Abdominal Trauma Imaging
WRITTEN BY: Nadia Mama, Hela Jemni, Nadia Arifa Achour, Ould Chavey Sidiya, Kaled Kadri, Mehdi Gaha, Ibtisem Hasni and Kalthoum Tlili
Published: August 14th, 2012
DOI: 10.5772/50426
FROM THE EDITED VOLUME
Abdominal Surgery
Edited by Fethi Derbel
Book Details Order Print
CHAPTER METRICS OVERVIEW
12,583 Chapter DownloadsView Full Metrics
1. Introduction
Isolated blunt abdominal trauma (BAT) represents about 5% of annual trauma mortality from blunt trauma. As part of multiple-site injury (polytrauma), BAT contributes another 15% of trauma mortality. In the abdominal trauma, the best exploration strategy is one that leads most quickly and reliably in the diagnosis of surgical injury. This strategy must be established based on hemodynamic status and clinical guidance but should never delay a therapeutic homeostasis. Early recognition and treatment decisions have been greatly impacted by increasingly sophisticated cross-sectional imaging and image-guided, minimally invasive therapies.
2. Imaging techniques
2.1. Plain radiographs
Plain x-ray plays a limited role in the evaluation of blunt abdominal trauma. Abdominal radiographs are usually unnecessary. X-rays of the chest and pelvis are often obtained to evaluate for concurrent thoracic or pelvic injuries. Abnormal chest x-ray findings of pneumothorax and rib fractures are associated with intraabdominal injuries and are indications for abdominal imaging if a mechanism for multisystem trauma is present. Common findings include free abdominal air (pneumoperitoneum), pneumothorax, and hemothorax. In the case of gunshot wounds, x-rays identify the location and number of retained projectiles.
2.2. Ultrasound
Ultrasound has become a common part of the initial assessment of blunt abdominal trauma. Ultrasound serves a screening function because it assesses for the presence of free fluid in the abdomen or pericardium but does not explicitly identify the source. The focused assessment with sonography in trauma (FAST) examination has been a standard part of the diagnostic algorithm since the 1990s in most U.S. trauma centers. The FAST exam looks for intra peritoneal and intra pericardial anechoic material representing fluid—which in the setting of trauma is assumed to be blood.
Advantages of ultrasound include portability (allowing it to be used during resuscitation), lack of ionizing radiation exposure, repeatability (allowing evaluation of changes in patient condition), and rapidity of the exam. Disadvantages include significant operator dependence and low sensitivity according detection of solid organ injury.
Ultrasound is considered most useful in detection of solid organ injuries with associated hemoperitoneum. It is considered insensitive for the detection of bowel or retroperitoneal injuries.
However, a recent study of Moriwaki and al [1] found ultrasound was 85% sensitive and 100% specific for detection of free air in a prospective study of 484 patients. Some small studies have also investigated the utility of ultrasound contrast agents to detect active bleeding [2]. Ultrasound is relatively sensitive for free abdominal fluid. In a study, continuous scanning of Morison’s pouch during infusion of DPL fluid revealed a mean detection limit of 619 mL. Only 10% of ultrasonographers (attending physicians and residents in emergency medicine, radiology, and surgery) detected volumes less than 400 mL. The sensitivity at 1 L was 97% [3]. Ultrasound is not sufficiently sensitive to exclude intraabdominal injury, limiting its utility as a definitive test for abdominal trauma. It allows selection of patients for CT and follow up.
2.3. Computed tomography in trauma
For most stable trauma patients, CT has become the definitive imaging modality of choice when intraabdominal injury is suspected. CT is rapid and highly sensitive and specific for many important injury types. The information provided by CT allows prognosis of injury and selective nonoperative management in both blunt and penetrating trauma. CT is less sensitive for some important injuries, including bowel and diaphragmatic trauma, a limitation that must be recognized to prevent clinical errors following a negative CT. For the evaluation of blunt abdominal or flank trauma, intravenous (IV) contrast should be routinely used, but oral contrast should not. We use 150 mL of intravenous contrast, infused at 2-4 mL per second, with CT being performed after a 60 second delay. A prior acquisition without intravenous contrast is recommended. It assesses solid organ hematomas and sentinel hematoma. Arterial acquisition is performed when chest exploration is indicated. Delayed acquisition (2-3 minutes) is performed when renal lesions or active bleeding are diagnosed. A number of studies have evaluated the safety and sensitivity of the triple-contrast CT approach. A metaanalysis performed by Goodman and al. [4], performed to determine the predictive value of CT for laparotomy in hemodynamically stable patients with penetrating abdominal trauma. They identified 180 studies but included only 5 because of methodologic concerns. The pooled sensitivity, specificity, negative predictive value, positive predictive value, and accuracy were 94.90%, 95.38%, 98.62%, 84.51%, and 94.70%, respectively.
Overall, triple-contrast CT appears to be a safe management strategy in highly selected stable patients without peritonitis on examination. Multiple studies have demonstrated the possibility of missed diaphragmatic injuries and, rarely, missed operative bowel injuries. Following a negative triple-contrast CT, observation or close follow-up should be ensured.
3. Lesional spectrum
3.1. Hemoperitoneum
Fluid is anechoic (black) on ultrasound. In the case of small amounts of hemoperitoneum, fluid may be visible only as an anechoic stripe separating the liver and the kidney on the right, or the spleen and the kidney on the left. Fluid may also accumulate between the spleen and diaphragm. Free fluid pooled in the pelvis is visible as anechoic collections lateral to the bladder on a transverse view. Free fluid may also be visible in the recto uterine recess (pouch of Douglas) in females using a sagittal view. In cases of gross hemoperitoneum, loops of bowel may be seen floating in blood.
Traumatic hemoperitoneum may be detected at CT anywhere in the peritoneal cavity.
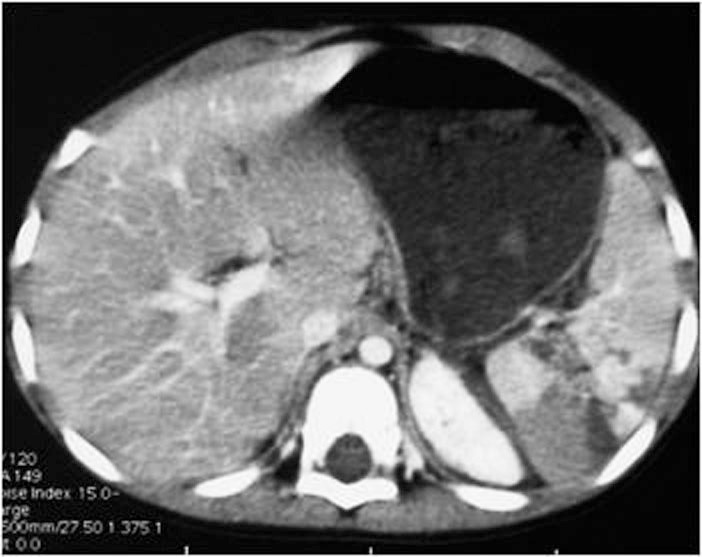
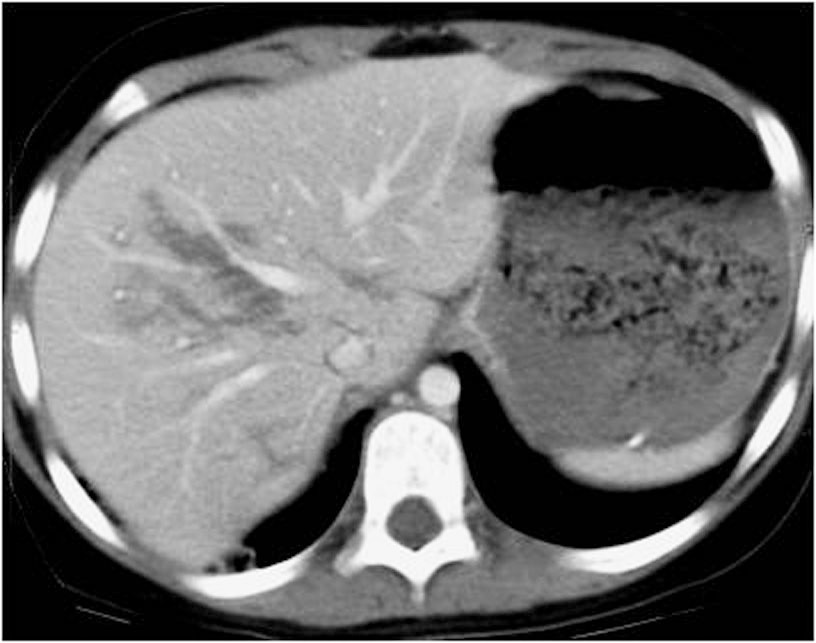
Measuring the CT attenuation of intraperitoneal fluid (figures 1, 2.) has proved exceedingly useful in its characterization, because intraperitoneal fluid collections in trauma patients may not always represent blood. Although there is variation with individual scanners, hemoperitoneum usually measures greater than 30 HU. By comparison, water-dense fluids in a trauma patient, such as ascites, urine, bile, or intestinal contents, measure 0 to 5 or 10 HU.
The recognition of water-dense fluids can be assisted by visual comparison with a fluid-filled structure, such as the gallbladder, or the soft tissue density of abdominal wall musculature; however, one may be misled by appearance only.
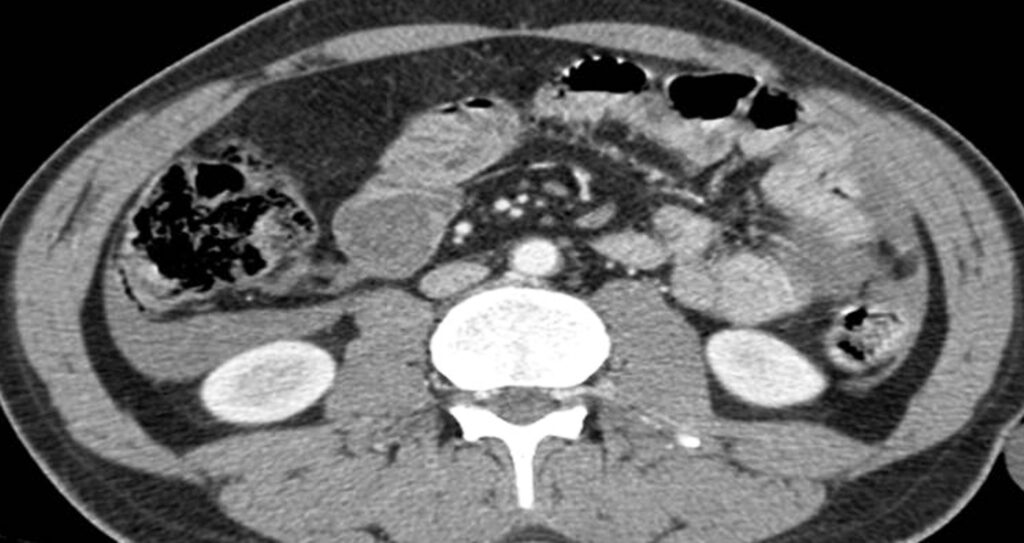
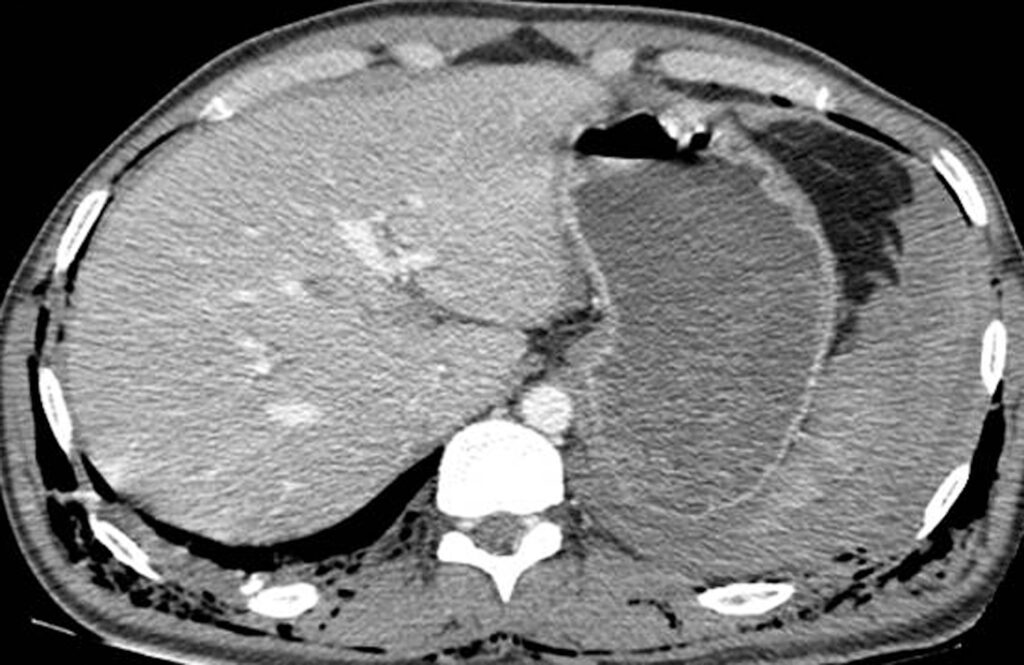
3.2. Pneumoperitoneum
Pneumoperitoneum is rare following blunt abdominal injury but can indicate bowel perforation. Soft-tissue windows are used at first, they can detect large amounts of Pneumoperitoneum who appear black (Figure 3.). Smaller collections are attempted on lung windows, followed by bone windows. When detected on CT, it is not specific for bowel injury because air tracking from thoracic injuries can collect in the abdomen. Following penetrating abdominal injury, Pneumoperitoneum detected on CT is likely to indicate bowel perforation and prompts laparotomy in most cases.
Pneumoperitoneum is also sometimes visible on ultrasound. Air is hyperechoic and disrupts the ultrasound beam, preventing visualization of deeper structures. Because bowel gas is normally present in the anterior midline abdomen, free air should be sought overlying the liver, where air is not normally present.
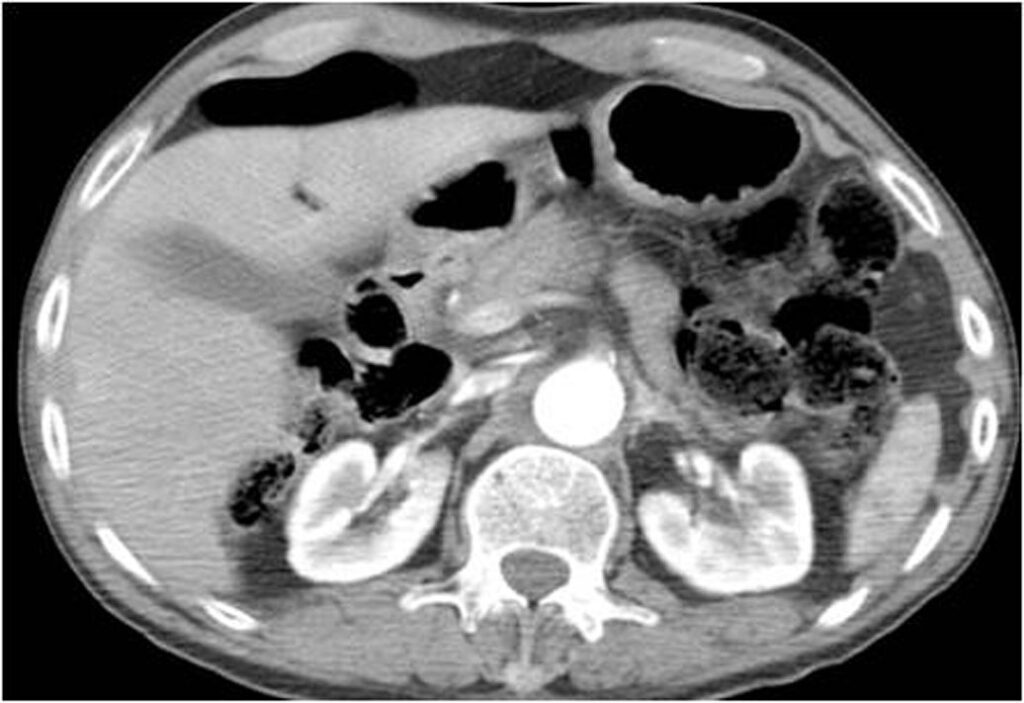
3.3. Active bleeding
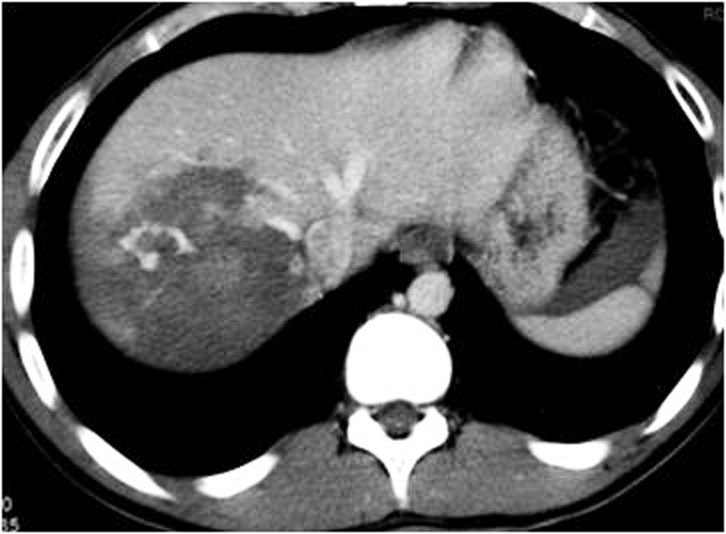
With the injection of contrast, active bleeding is visible as a bright white “blush” or amorphous collection on arterial phase imaging within a hypodense injured solid organ indicates active bleeding. This must be distinguished from normal enhancement of vessels within solid organs, such as portal and hepatic vessels within the liver. On delayed imaging the area of active extravasation remains high in attenuation and increases in size (Figure 4.), a result of ongoing bleeding from the injured vessel after the initial phases of scanning.
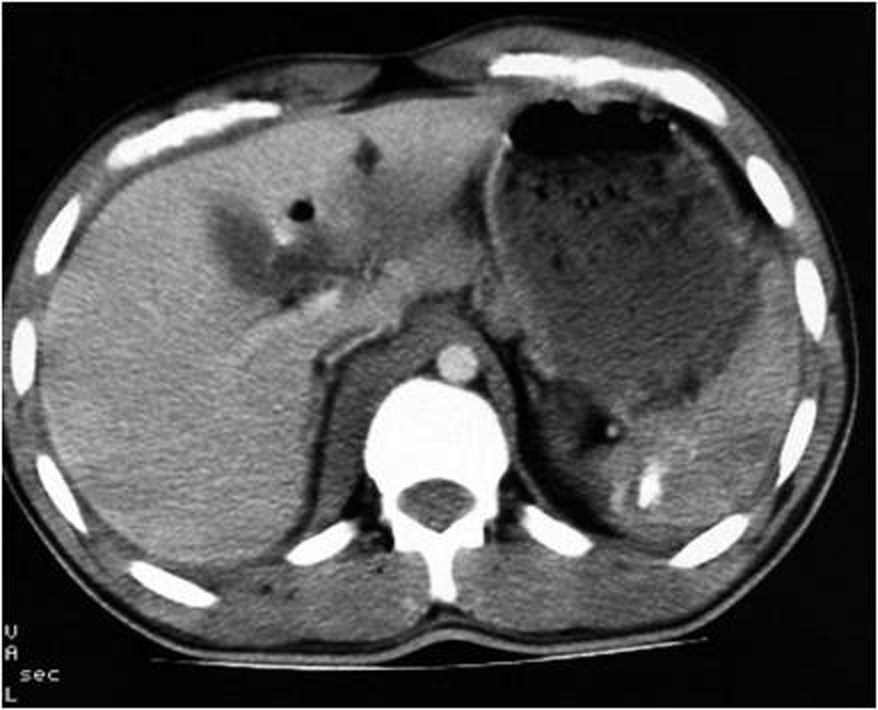
3.4. Lacerations
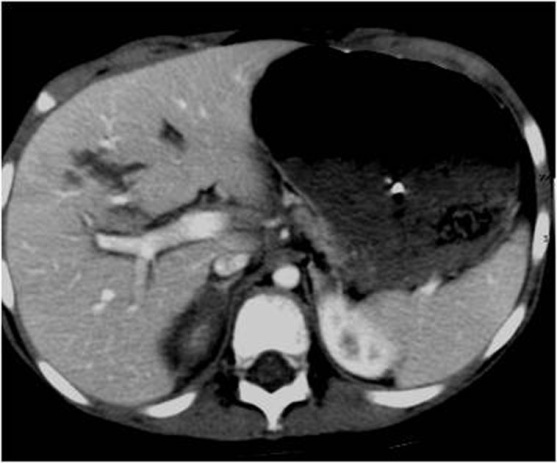
The laceration may initially be difficult to recognize in sonography or may appear slightly echogenic band. Acute splenic lacerations are seen on contrast-enhanced MDCT as linear or branching areas of low attenuation with well-defined margins (Figure 5.). When lacerations extend through the organ capsule, hemoperitoneum results; if the capsule is intact, a subcapsular hematoma may be demonstrated. With time, the lacerations decrease in size and number. The margins become less well defined, and the area becomes isodense compared with normal splenic parenchyma. Although healing changes may be seen within 2 to 3 days, complete resolution may take weeks to months, depending on the size of the original injury. An increase in the number of lacerations on follow-up MDCT should alert the radiologist to the possibility of injury progression, and close clinical follow-up with MDCT or angiography is advised. Splenic clefts may mimic lacerations on MDCT but typically have smooth or rounded margins. Fat may be periphery and become less visible, splenic clefts remain unchanged in appearance on delayed images.
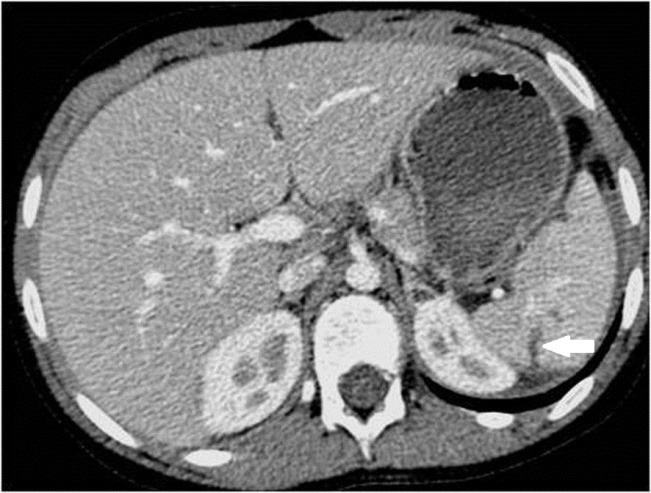
3.5. Contusions
They represent areas of injury. They appear on contrast-enhanced CT as parenchymal areas of low attenuation with irregular edges (Figure 6.). Contusions are invariably a minor injury and gradually decrease in size as the injury heals.
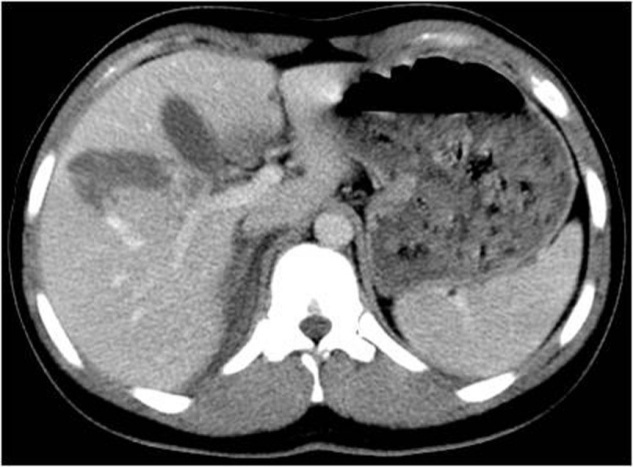
3.6. Fractures
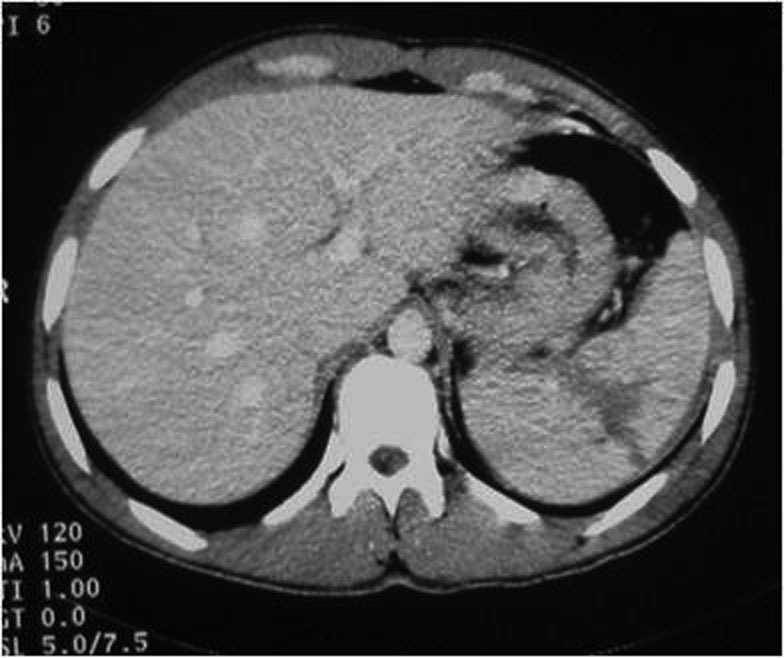
- When the bands of laceration cross the hypodense parenchyma, joining two opposite edges through the hilum, they are called fracture (Figures 7., 8.).
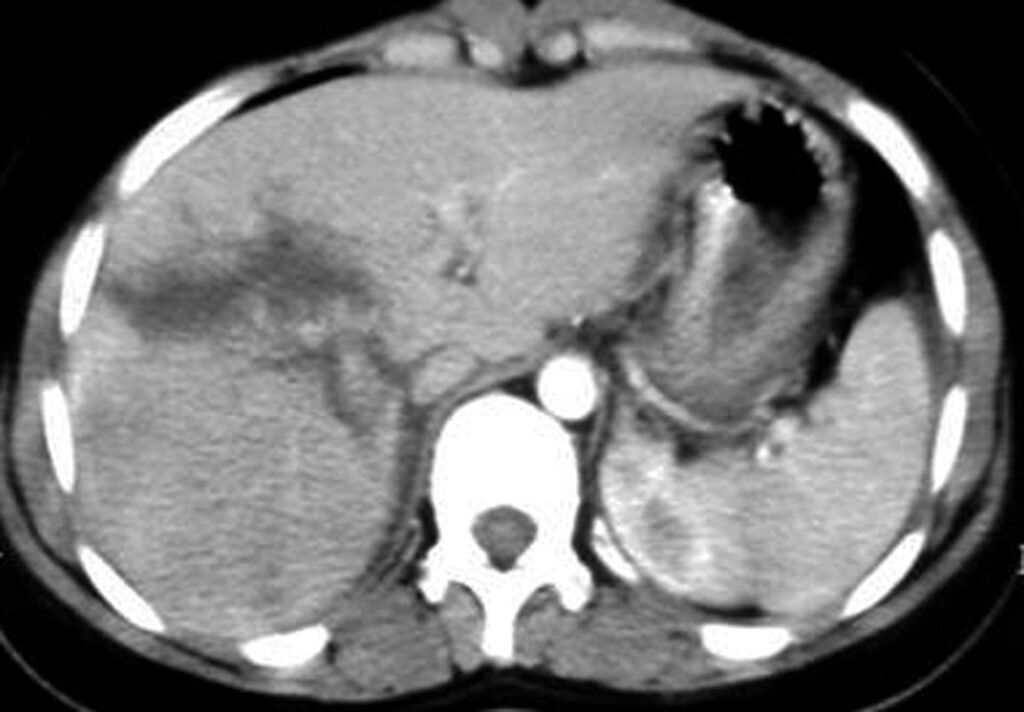
3.7. Hematoma
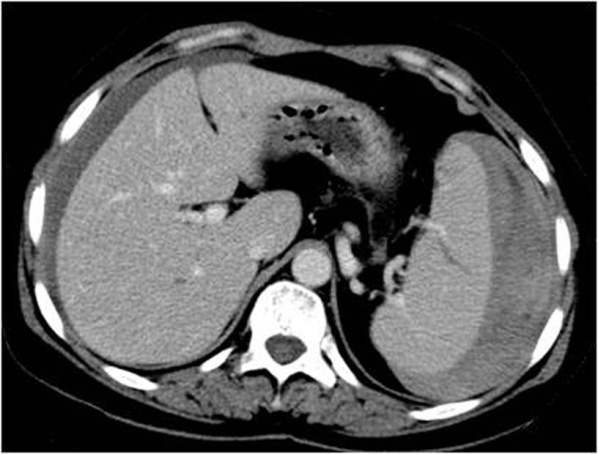
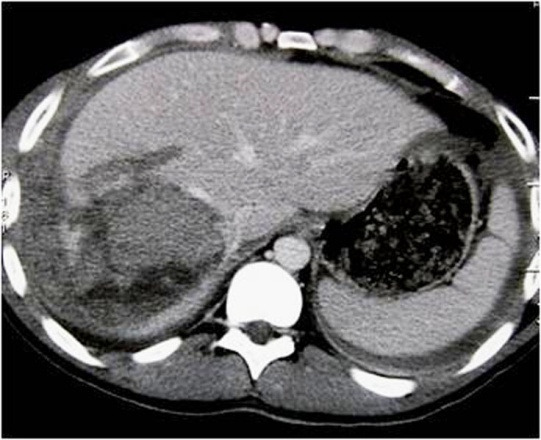
- Subcapsular hematomas: appear as crescentic regions of hyperdensity compared with adjacent normal parenchyma. After contrast administration, subcapsular hematomas are seen as a low-attenuation collection between the splenic capsule and enhancing splenic parenchyma that compress the underlying contrast-opacified organ parenchyma (Figure 9.). This finding is useful in differentiating subcapsular hematomas from free intraperitoneal fluid or blood. In sonography, it appears as a hyperechoic or hypoechoic rim or crescent
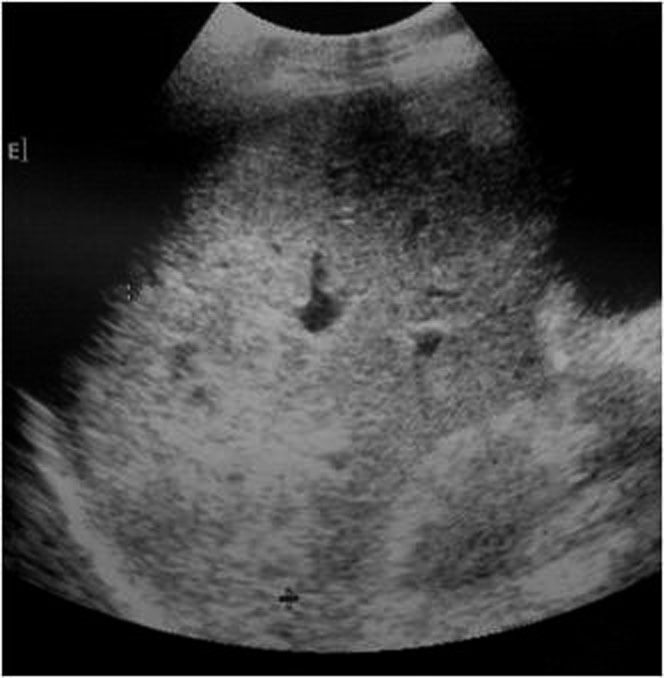
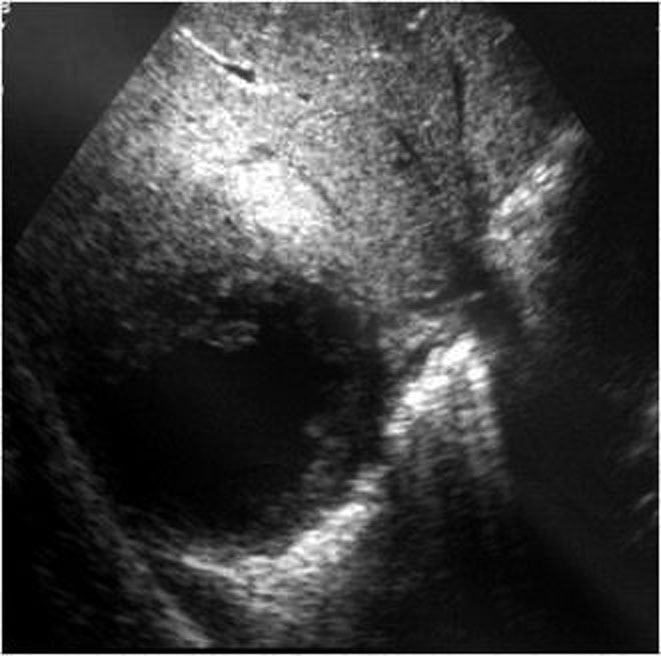
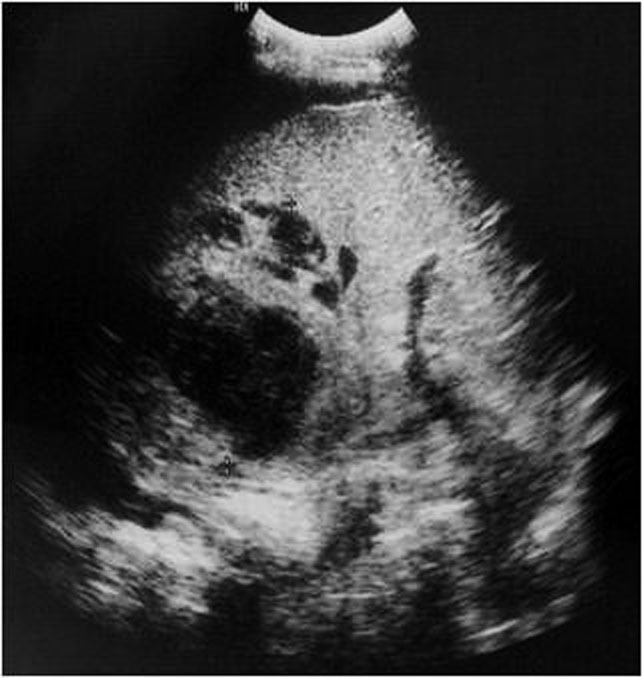
- Intraparenchymatic hematomas: appear as a round hyperdensity compared with adjacent normal parenchyma. After contrast administration, they appear as low-attenuation zones within the parenchyma; these may be homogeneous or inhomogeneous (Figure 10.). On sonography, they are present as a localized area of increased echogenicity (Figures 11., 12., 13.).
3.8. IVC shock
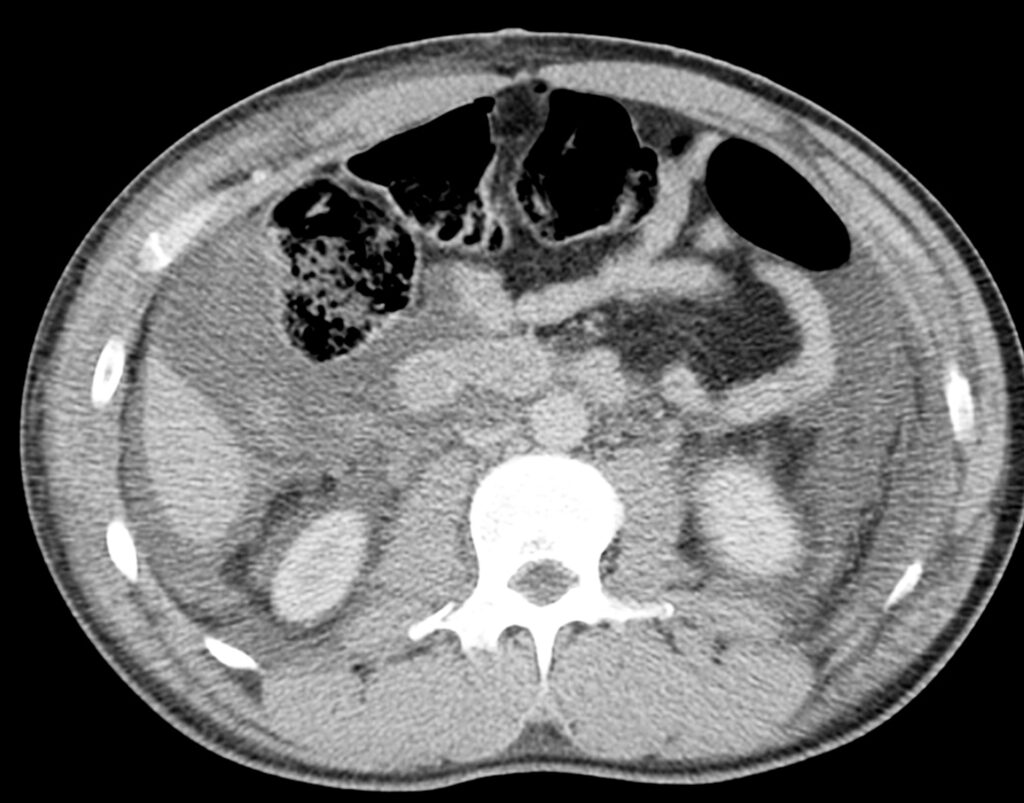
In cases of severe volume depletion (generally from hemorrhagic shock following trauma), the infrahepatic inferior vena cava (IVC) appears flattened. This appearance can occur in patients before the development of clinical hypotension or hemodynamic collapse and demands immediate volume resuscitation (figure 14.).
Shock bowel is a term for secondary bowel injury resulting from sustained systemic hypotension. The CT appearance includes diffuse bowel wall thickening visible on CT.
CT hypotension complex associates multiple findings: Shock bowel with IVC and aortic flattening, abnormal pancreatic enhancement and peripancreatic fluid, and poor enhancement of the spleen and liver because of hypotension.
4. Spleen injury
The spleen is the intra-abdominal organ most often injured as a result of blunt trauma. The spleen is the most vascular organ of the body and, for this reason, splenic injury is potentially life threatening. The most common mechanism for such injury is motor vehicle collision. Left lower rib fractures are suggestive of spleen injury, although an intact rib cage does not exclude spleen trauma. The other trauma mechanisms are penetrating trauma stab, iatrogenic trauma following colonoscopy and spontaneous spleen rupture in some diseases that involve the spleen like infectious mononucleosis, hemopathies or metastasis. Nonsurgical management is becoming the preferred treatment method for adult patients (with blunt splenic injuries) who are hemodynamically stable [5].
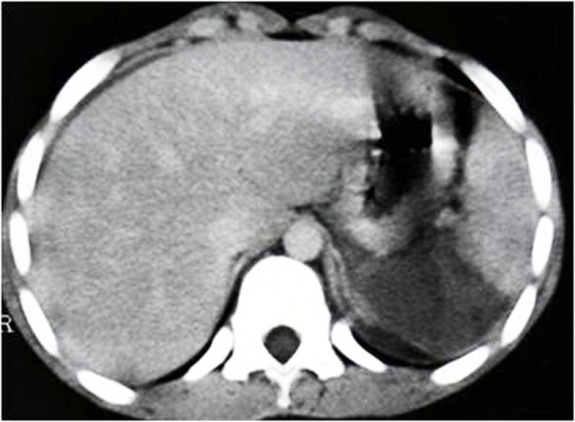
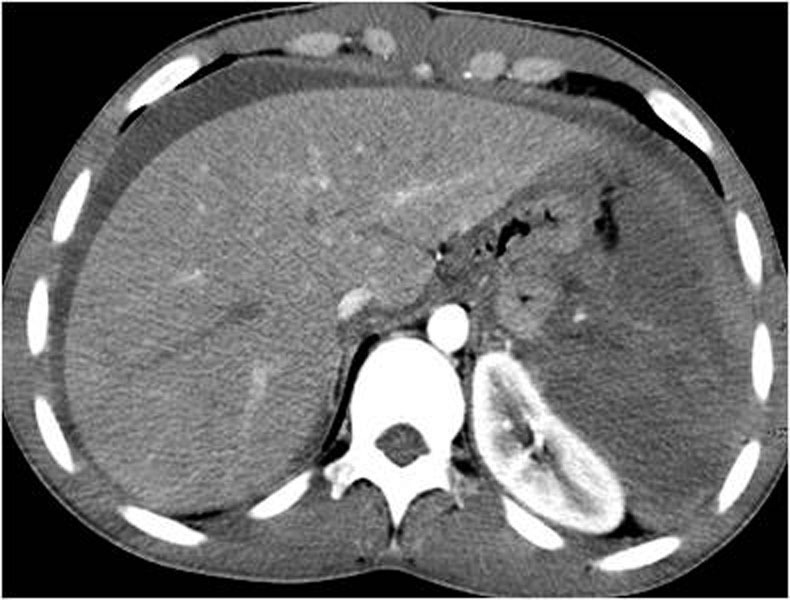
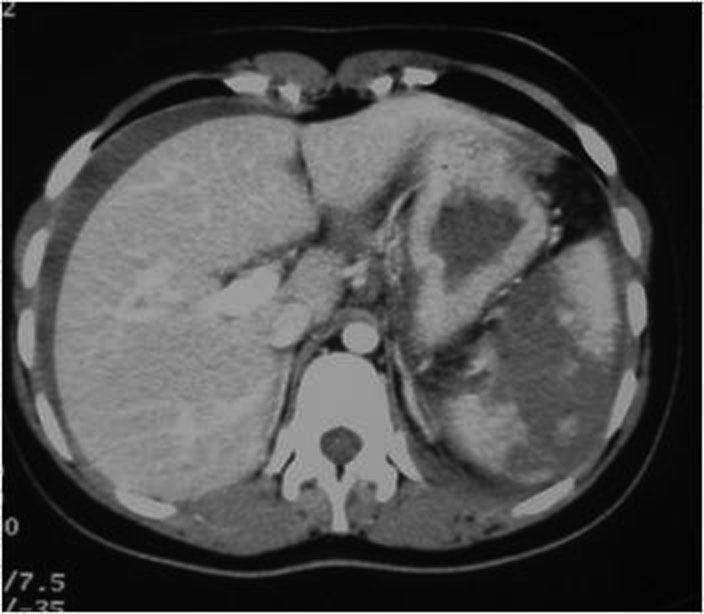
Ultrasonography is a quick and noninvasive technique for detecting intra-abdominal blood. When hemoperitoneum is present and mainly when it is peri splenic, it highly suggests spleen trauma. However, a high number of significant abdominal organ injuries occur without associated hemoperitoneum. A large retrospective study performed at the University of Maryland Shock Trauma Center (UMSTC) showed that 57 (27%) of 210 splenic injuries were found to have no hemoperitoneum on admission computed tomography (CT) [6]. The Doppler color does not improve US performances. Contrast enhanced ultrasound seems to be a promising technique. MDCT is highly accurate (98%) in diagnosing splenic injury [7]. It is important to image for splenic injury during the portal-venous phase, because heterogeneous enhancement in the early arterial phase may simulate injury. The arterial phase is useful in differentiating between active arterial bleeding and posttraumatic vascular injuries, including pseudoaneurysm and traumatic arteriovenous fistulae. The principle types of splenic injury include hematoma, laceration, active hemorrhage, posttraumatic splenic infarct, and vascular injuries, including posttraumatic pseudoaneurysms and arteriovenous fistulae [8], (figures 5., 8., 15., 16., 17. 18., 19.).
Spleen injuries are graded in severity based on CT appearance using a five-point scale (Table1.) according to AAST scaling [9, 10, 11]. Grading of splenic trauma serves many purposes, even if it cannot reliably be used as a prognostic indicator when nonoperative management is chosen. Marmery et al. state, “The purpose of a grading system is to standardize reporting, plan appropriate management, and enable comparisons between institutions and studies [11]. We must note that the presence of splenic vascular injuries is a predictor of failure of nonoperative management that is not explicitly defined under the 1987 original or 1994 revised AAST splenic trauma grading system; Marmery et al. promote their alternative grading system in which vascular splenic injuries are better defined (table2).
| Spleen | Injury type | Description of injury | AIS |
| I | Hematoma | Subscapular, <10 % surface area | 2 |
| laceration | Capsular tear < 1 cm parenchymental depth | 2 | |
| II | Hematoma | Subscapular, 10 to 50 % surface area intraparenchymaental < 5 cm in diameter | 2 |
| laceration | Capsular tear, 1 to 3 cm parenchymental depth, that does not involve a trabecular vessel. | 2 | |
| III | Hematoma | Subscapular, “/> 50 % surface area or expending; ruptured subscapular or parenchymental hematoma; intraparenchymental hematoma”/> 5 cm or expending. | 3 |
| laceration | “/> 3 cm parenchymental depth or involve a trabecular vessel. | 3 | |
| IV | laceration | Laceration involving segmental or hilar vessels producing major devascularization ( “/> 25 % of spleen) | 4 |
| V | hematome | Completely shattered spleen | 5 |
| laceration | Hilar vascular injury devascularizes spleen | 5 |
Alternate grading system for splenic trauma [11].
| Grade | criteria | |
| 1 | Subscapular hematoma < 1cm thick Laceration < 1cm parenchymal depth Parenchymal hematoma < 1cm diameter | |
| 2 | Subscapular hematoma 1-3 cm thick Laceration 1-3 cm parenchymal depth Parenchymal hematoma 1-3 cm diameter | |
| 3 | Splenic capsular disruption Subscapular hematoma “/>3 cm thick Laceration “/>3 cm parenchymal depth Parenchymal hematoma “/>3 cm diameter | |
| 4 | 4a | Active intraparenchymal and subcapsular bleeding Splenic vascular injury (pseudoaneurysm or arteriovenous fistula) Shattered spleen |
| 4b | Active intra peritoneal bleeding | |
Alternate grading system for splenic trauma in which vascular injuries are better defined [11].
5. Liver injury
The liver is frequently injured in blunt trauma. The prevalence of liver injury in patients who have sustained blunt multiple trauma has been reported to be 1%–8% [12]. However, liver injuries can be detected in up to 25% of patients with blunt trauma if whole-body computed tomography (CT) is performed as the initial diagnostic procedure in severely injured patients admitted to the trauma center. Isolated hepatic lesions are rare and in 77–90% of cases, lesions of other organs and viscera are involved [13]. Blunt liver trauma still carries a significant morbidity and mortality. The reported mortality rate attributable to blunt liver injury ranges from 4.1% to 11.7% [12, 14, 15].
Detected lesions are the consequence of 3 different mechanisms: sudden deceleration such as in crash-car events, direct impact or penetrating wound. The more involved site is the right lobe, posterior–superior segments particularly, because it is the more voluminous portion of the liver; posterior superior hepatic segments are proximal to fixed anatomical structures such as ribs and spine, that may have an important role in producing the lesion. Coronal ligamentous insertion in this region increases the effect of the acceleration–deceleration mechanism. Associated lesions usually are homolateral costal fractures, lesions of the inferior right pulmonary lobe, haemothorax, pneumothorax, renal and/or adrenal lesions [16].
Traumatic lesions of the left hepatic lobe are rare and usually associated with direct impact of the superior abdomen. Associated lesions with left hepatic lobe injuries include sternal fractures, pancreatic, myocardial, duodenal and transverse colon lesions [16]. Lesions of the caudate lobe are extremely rare, usually not isolated and are found with other significant lesions.
Generally, hemodynamically stable patients are submitted to sonographic examination for detection of fluid collections and, possibly, of parenchymal lesions. Sonographic findings of a traumatic lesion or of peritoneal fluid are an indication for CT examination. Patients in critical clinical condition go directly to CT examination of the abdomen and pelvis. CT with IV contrast is highly sensitive for liver injuries.
As reported in literature [9, 17, 18].
Radiological findings of traumatic lesion of the liver are: lacerations (Figure 20.), contusions, subcapsular/central hematoma, active hemorrhage (figure 10., figure 21.), periportal tracking (Figure 22.), juxtahepatic venous injuries and avulsion of the hepatic pedicle.
Hepatic lacerations are the most common type of parenchymal liver. Lacerations can be classified as superficial (<3 cm in depth) or deep (>3 cm) [19]. Lacerations that extend to the postero superior region of segment VII may be associated with retroperitoneal hematomas around the IVC and accompanied by adrenal hematoma [20]. Lacerations that extend to the hepatic hilum are commonly associated with bile duct injury and are thus likely to lead to the development of a biloma. Lacerations and fractures that involve segment VI and VII follow venous path and can be extend into one or more major hepatic veins or the IVC. Such lesions are considered as major hepatic venous injuries can be life threatening and therefore are an indication for surgical treatment [21, 22]. When a fracture is detected, we must assess the non vascular excluded parenchyma. Large acute intraparenchymatic hematoma may be associated with perfusion trouble secondary to tissue compression and ischemia.
The detection of active contrast material extravasation at CT is important because it indicates an ongoing, potentially life-threatening hemorrhage. Several investigators clearly demonstrated that active contrast material extravasation at contrast-enhanced CT is a strong predictor of failure of nonsurgical management and recommended prompt surgical or angiographic intervention [23-25].
Periportal low attenuation results as regions of low attenuation that parallel the portal vein and its branches on CT scans. Periportal low attenuation seen in proximity to a hepatic laceration may represent a hemorrhage dissecting into the periportal connective tissue. However, it can also be due to distention of the periportal lymphatic vessels secondary to elevated central venous pressure (after massive intravenous filling, high abundance pneumothorax, or pericardial tamponade [26]. Patients with periportal low attenuation without evidence of significant parenchymal injury can be successfully treated conservatively [19].
Liver injuries are graded in severity based on CT appearance using a six-point scale (Table 3) according to AAST scaling that guides nonoperative management. This scaling is regarding the lesion extension and bleeding [9]. The AAST injury grading scale includes some criteria that cannot be assessed with CT, CT findings generally leading to underestimation of injury severity.
| Liver | Injury type | Description of injury | AIS |
| I | Hematoma | Subscapular, <10 % surface area | 2 |
| laceration | Capsular tear < 1 cm parenchymental depth | 2 | |
| II | Hematoma | Subscapular, 10 to 50 % surface area intraparenchymaental < 10 cm in diameter | 2 |
| laceration | Capsular tear, 1 to 3 cm parenchymental depth, < 10 cm length | 2 | |
| III | Hematoma | Subscapular, “/> 50 % surface area of ruptured subscapular or parenchymental hematoma ;intraparenchymental hematoma”/> 10 cm or expending. | 3 |
| laceration | Capsular tear “/> 3 cm parenchymental depth | 3 | |
| IV | laceration | Parenchymal disruption involving 25 to 75 % hepatic lobe or 1 to 3 Couinaud’s segments | 4 |
| V | laceration | Parenchymal disruption involving “/> 75 % hepatic lobe or “/> 3 Couinaud’s segments within a single lobe | 5 |
| vascular | Juxtahepatic venous injuries, ie, retrohepatic venacava/ central major hepatic veins | 5 | |
| VI | vascular | Hepatic avulsion | 6 |
Alternate grading system for liver trauma [11].
Delayed CT features: delayed complications detected at follow-up CT has increased with non surgical management of liver injuries. These posttraumatic complications include delayed hemorrhage, abscess, posttraumatic pseudoaneurysm and hemobilia, and biliary complications such as biloma and bile peritonitis and are more common in patients with severe, complex liver injuries.
6. Renal trauma
Urinary tract injury occurs in 10% of all abdominal trauma patients. Mechanisms of renal injuries result from Blunt renal and accounts for up to 80%–90% of all cases; Penetrating trauma accounts for approximately 10% of all renal injuries caused by gunshot or stab wounds except for the few iatrogenic injuries resulting from renal biopsy [27]. There is a broad consensus in favor of less invasive procedures and conservative management when a patient is stable except in cases of severe injury such as pedicle lesion or complex laceration of uretro-pelvic junction.
Contrast material–enhanced computed tomography (CT) is the imaging modality of choice in the evaluation and management of renal trauma. It can quickly and accurately depict renal injuries as well as associated injuries to other abdominal or retroperitoneal organs. It demonstrates the extent of damage tissue, perirenal hemorrhage, extravasation of urine and renal pedicle or vascular injuries. CT is important for optimal evaluation and both physiologic and morphologic information and can be obtained by using CT with contrast enhancement. The CT protocol for suspected renal trauma includes an initial arterial phase with a scanning delay of 20–30 s to identify vascular damage, followed by a nephrographic phase at 70–80 s to identify parenchymal lesions and a possible late phase at 3–20 min to detect lesions to the urinary tract [27-30].
The use of MRI imaging is limited in acute renal trauma because of accessibility, motion artifacts, and the much longer scaning time than with CT.
Like for the other visceral injuries, various classification systems have been devised but the grading system of the (AAST) is widely accepted and used (table 4) [27, 30].
| kidney | Injury type | Description of injury | AIS |
| I | contusion | Microscopic or gross hematuria, urologic studies normal | 2 |
| hematoma | Subscapsular, nonexpanding without parenchymal laceration | 2 | |
| II | Hematoma | Nonexpending perirenal hematoma confirmed to renal retroperitenum | 2 |
| laceration | < 1.0 cm parenchymal depth of renal cortex without urinary extravasation | 2 | |
| III | laceration | “/>1.0 cm parenchymental depth of renal cortex without collecting system rupture or urinary extravasation. | 3 |
| IV | laceration | Parenchymal Laceration extending through renal cortex , medulla , and collecting system | 4 |
| vascular | Main renal artery or vein injury with contained hemorrahge | 4 | |
| V | laceration | Completely shattered kidney | 5 |
| vascular | Avulsion of renal hilum that devascularizes kidney | 5 |
The American association for the surgery of trauma (AAST) renal injury severity scale [29].
6.1. Grade 1 injuries
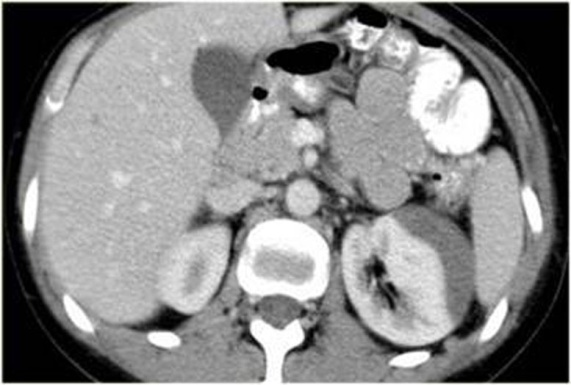
Contusions in this grade are visualized as poorly marginated round or ovoid areas of decreased enhancement and a delayed or persistent nephrogram compared with normal kidney; No evidence of contrast extravasation would be seen in the excretory phase, since the collecting system is not involved. It constitutes 75%–85% of all renal injuries in most series [30]. These category injuries are usually managed conservatively. A small subcapsular haematoma appears as a hypodense lesion flattening the renal capsule (Figure 23.). Other findings would include small subsegmental cortical infarcts (small wedge-shaped, well-defined hypodensity) and limited perinephric haematoma.
6.2. Grade II and grade III injuries
These grade include non expanding perinephric hematomas confined to the retroperitoneum and superficial cortical lacerations measuring less than 1 cm in depth (grade II) or more than 1 cm (grade III) without involvement of the collecting system (Figure 24.); that extend into the medulla [29, 30].
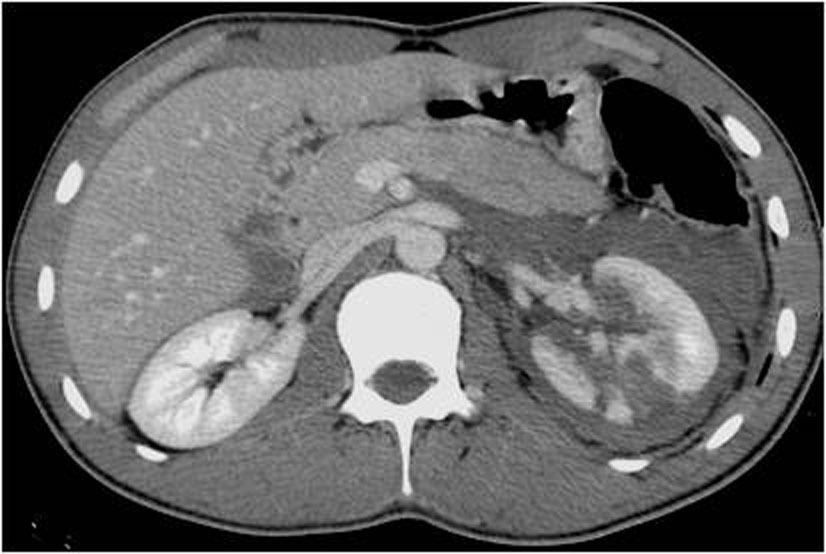
6.3. Grade IV injuries
Comprise cortical-medullary lacerations extending to the collecting system and injuries to the renal artery and vein with contained haemorrhage. A topographical criterion for CT recognition of injury to the calyceal system is the detection on delayed postcontrast CT images of urinary extravasion in the posterolateral perirenal space (Figures 25., 26.), in contrast to what happens in injuries to the renal pelvis, ureteropelvic junction or ureters, in which the urine typically collects medially at times along the course of the ureter. Urinary extravasation alone is not an indication for surgical exploration; it resolves spontaneously in approxymately 80 of cases. This grade includes segmental infarctions caused by thrombosis dissection or laceration of the segmental arteries.
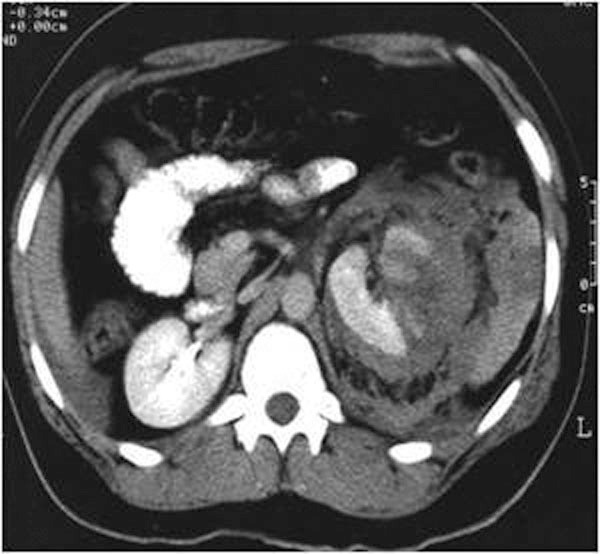
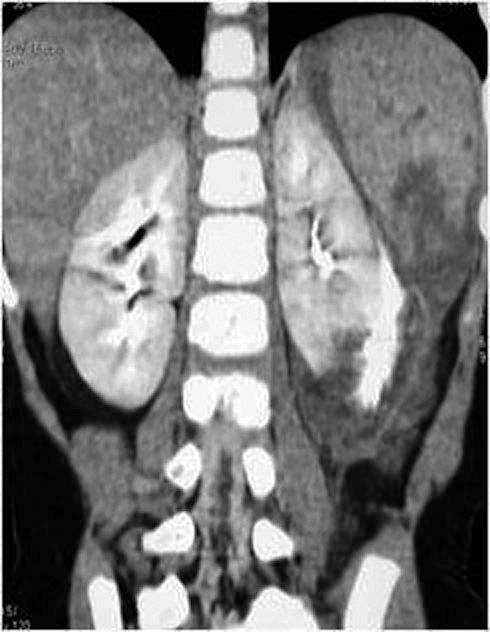
6.4. Grade V injuries
It represent the most severe type of renal trauma and include shattered kidney which is rupture into three or more separate fragments, partial tears or complete laceration (avulsion) of the ureteropelvic junction, and thrombosis of the main renal artery or vein with devascularization of the kidney. The absence of the nephrographic effect and the presence of an extensive retroperitoneal haematoma (Figure 27.), especially in medial location, should raise suspicion of injury to the vascular pedicle. A typical finding is devascularisation, but more in general in arterial infarctions, is the so-called “rim sign”, which is due to opacification of the capsule and subcapsular parenchyma by intact collateral capsular vessels.
Conservative management may also have a role in grade V injuries, with nephrectomy being performed in only 22% cases of major vascular trauma, in some cases deferred to 21 days after injury. It is evident that the only absolute indication for immediate exploratory surgery is the presence of “uncontrollable” active bleeding [29, 30].
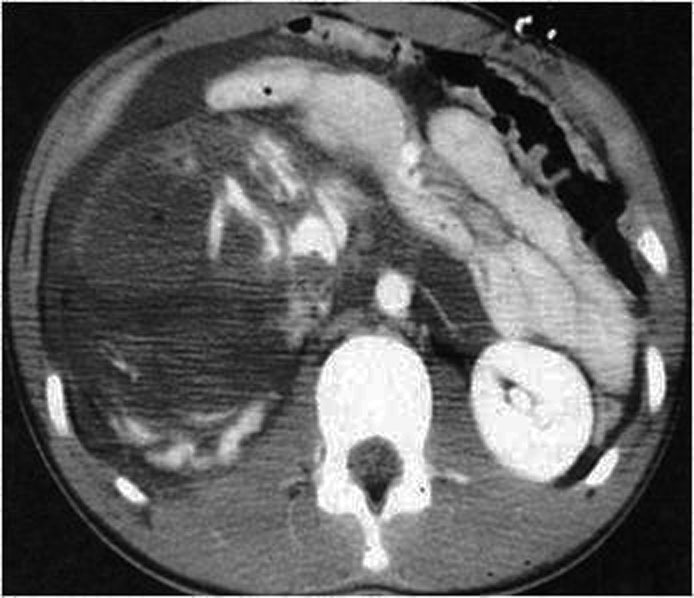
6.5. Iatrogenic renal trauma
Ultrasound-guide percutaneous core-needle biopsy is a frequently used for diagnosis of renal parenchymal disease. Biopsy complications including perirenal hematoma laceration of the renal arterial branch, arterioveinous fistula and pseudoaneurysm may occur. The majority of acquired renal arteriovenous fistulae resulting from renal biopsy heal spontaneously. Angiography can be performed effectively to achieve hemostasis.
Renal vascular injury can occur during angiography like renal artery angioplasty or a stenting procedure.Extracorporeal shock-wave litrotripsy is treatment and can lead to perirenal hematoma, (15% to 30%of cases) rupture of the kidney and lacerations [29, 30].
6.6. Complications of renal trauma
Complications occur in 3% to33% of patients with renal trauma and include urinary extravasation with urinoma, infected urinoma, perinephric abcess, secondary hemorrhage secondary to a rupture of arteriovenous fistula or pseudoaneurysm.
Late or delayed complications of renal trauma develop more than 4 weeks after injury and include hypertension, hydronephrosis, calculus formation, and chronic pyelonephritis, arterioveinous fistula.
The term Page kidney refers to hypertension secondary to constrictive ischemic nephropathy caused by large chronic subcapsular hematomas, which exert a mass effect on the adjacent renal parenchyma, indenting or flattening the renal margin. [27, 29].
7. Pancreatic injury
Pancreatic injuries are rare, occurring in around 2% of blunt trauma patients [31], but may be associated with high morbidity and mortality, particularly if diagnosis is delayed. Indeed, the probability of complications after duodenal or pancreatic trauma ranges between 30% and 60%. Hence, early diagnosis is critical. These injuries often occur during traffic accidents as a result of the direct impact on the upper abdomen of the steering wheel or the handlebars. localisation pancreatic injuries are rarely isolated; Organ injuries most commonly associated are hepatic (46.8% of cases), gastric (42.3%), major vascular (41.3%), splenic (28.0%), renal (23.4%), and duodenal (19.3%) [32].
Pancreatic injuries are often subtle and may be overlooked in patients with extensive multiorgan trauma. In 20%–40%, initial CT findings of patients with pancreatic injuries may be within normal limits in the first 12 hours after the injury [33, 34]. It is important to detect disruption of the pancreatic duct which is treated surgically or by therapeutic endoscopy with stent placement, while injuries without duct involvement are usually treated nonsurgically.
Today, computed tomography (CT) provides the safest and most comprehensive means of diagnosis of pancreatic injury in hemodynamically stable patients.
Serum amylase or lipase activity can be raised although in up to 40% it remains normal. Repeated testing is recommended, but results do not indicate the severity of the injury [32].
7.1. CT findings in pancreatic injury
The absence of a pancreatic parenchymal phase (35–40-second delay) in whole-body CT is an obvious limitation for lesions detection. The CT findings of acute pancreatic trauma (PT) may be separated into specific (direct) features and nonspecific (indirect) features (table5) [31, 35, 36].
| Pancreas | |
| Direct findings | Secondary findings |
| Pancreatic enlargement Laceration (focal linear nonenhancement) Comminution Inhomogeneous enhancement | Peripancreatic fat stranding Peripancreatic fluid collections, which may communicate with a laceration Fluid between the splenic vein and pancreas Hemorrhage Thickening of the left anterior pararenal fascia Associated injuries to adjacent structures |
CT findings in pancreatic injuries due to blunt trauma [31].
Initial CT examination can appear normal. It is postulated that these false negative findings may result from obscuration of the fracture plane, surrounding hemorrhage, or close apposition of the pancreatic fragments [37]. Direct CT signs of PT include evidence of parenchymal laceration, transaction and focal enlargement or hematoma. Lacerations can be classified into superficial laceration (involving <50% of the parenchymal thickness) and deep laceration (>50% pancreatic parenchyma) (figure 28.); Using this cutoff can help detecting pancreatic duct disruption (figure 29.). It has been shown that main duct disruption was likely present in cases of deep laceration or complete transection of the pancreatic parenchyma [38]. Active hemorrhage is sometimes seen. Contained vascular injuries, such as pseudoaneurysms, may also be identified on CT as evidenced by focal hyperattenuating areas which are seen to wash out on delayed phases of image acquisition (table 6) [37].
| grade | Injury | Description |
| I | hematoma | Minor contusion without duct injury |
| laceration | Superficial laceration without duct injury | |
| II | Hematoma | Major contusion without duct injury |
| laceration | Major laceration without duct injury | |
| III | laceration | Distal transaction or parenchymal injury with duct injury |
| IV | laceration | Proximal transaction or parenchymal injury involving the ampulla or bile duct |
| V | disruption | Massive disruption of the pancreatique head |
Scoring pancreatic injury [31].
The indirect signs tend to be less specific when used to assess the presence of pancreatic trauma. These indirect imaging findings include peripancreatic and fat stranding and hemorrhage [32, 39]. Fluid between the splenic vein and the pancreas also suggests pancreatic injury. It has been shown in 90% of verified cases of blunt pancreatic trauma [40]. However, this finding is nonspecific.Verifying ductal status is one of the most important predictors of outcome in pancreatic trauma [41-43]. Any delay in diagnosis of major duct injury can result in a significant increase in mortality and direct complications such as fistula, abscess, and pseudocyst (figure 30).
However, the pancreatic duct is inconsistently visualized on CT scan. New studies has shown that thinner collimation and post-processing techniques such as multiplanar reformations can improve pancreatic duct visualization [44, 45].
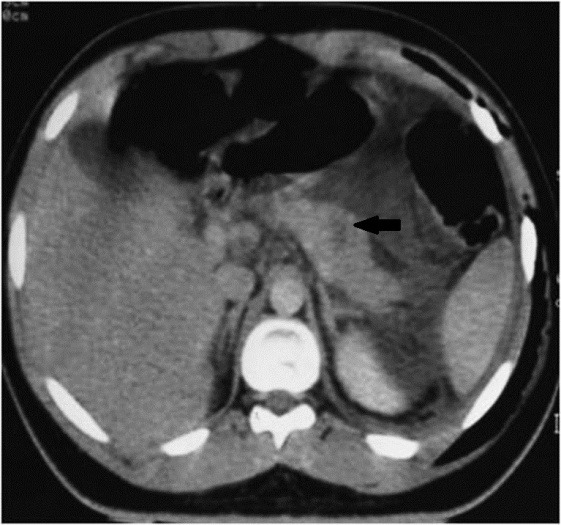
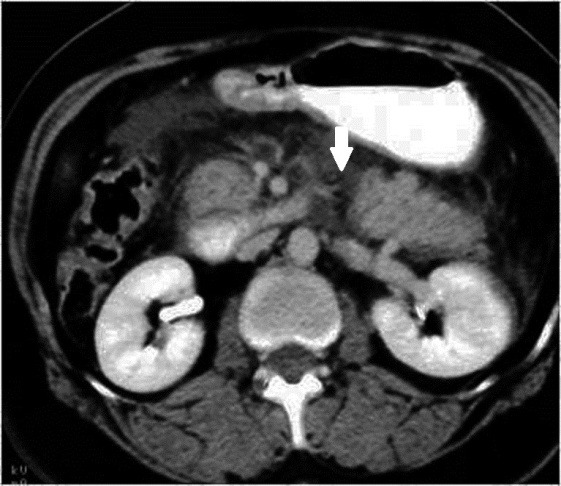
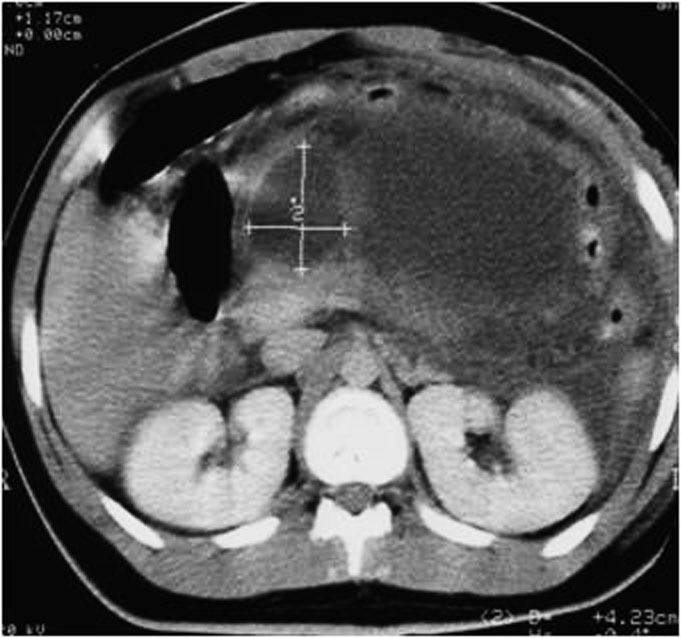
Trauma: Axial contrast-enhanced CT scan shows two large loculated fluid collections
7.2. Endoscopic Retrograde Cholangiopancreatography (ERCP)
Has been traditionally the gold standard for imaging of the pancreatic duct because of its potential to provide diagnostic images and to direct image-guided therapy. However, in the trauma setting, ERCP may not be readily available or feasible. ERCP is indicated when pancreatic injuries are detected at CT or MR imaging or if there is high clinical suspicion of ductal injury. ERCP can direct appropriate surgical repair or can be used for primary therapy by means of stent placement [31, 46].
7.3. Magnetic resonance cholangiopancreatography (MRCP)
Has proven a useful tool for diagnosing various abnormalities affect the pancreas and pancreatic duct. It has the ability to visualize not only the duct but also the pancreatic parenchyma and the surrounding environment [37]. The extension of a fracture to involve the pancreatic duct may more clearly be identified. MRI has also been found to be particularly useful in follow up of conservatively managed parenchymal injuries, fluid collections, and minor duct abnormalities [47]. MR follow-up plays a large role in young patients and children where minimizing cumulative radiation dose is of particular importance.
MRCP, in combination with the intravenous administration of secretin, increases pancreatic exocrine output, consequently better duct distension and delineation [48, 49]. Pertaining to blunt pancreatic trauma, secretin MRCP has been shown to be safe and useful in providing additional information on ductal disruption, facilitating subsequent management decisions [49].
8. Biliary tract injury
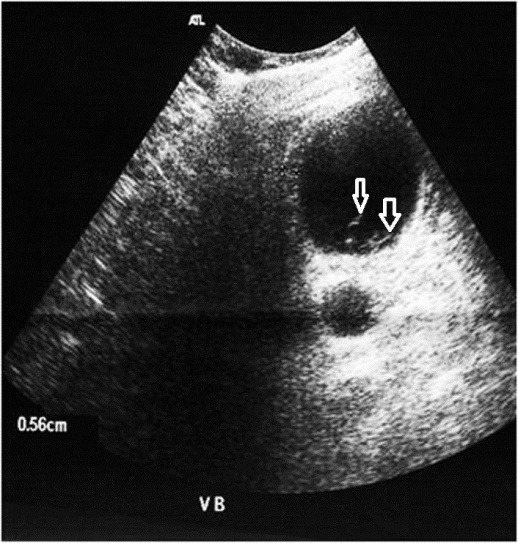
Biliary tract injuries are rare following blunt trauma, occurring in only around 2% to 3% of patients undergoing laparotomy [31]. The most common location of biliary injury is the gallbladder, followed by the common bile duct and the intrahepatic ducts. Injuries to the gallbladder may be classified into one of three main categories: contusion, laceration/ perforation, or complete avulsion. Gallbladder injuries are difficult to recognize because of the common association to adjacent organ injury. A collapsed gallbladder or thickening (figures 31, 32) or disruption of the gallbladder wall suggests injury but none of these signs is specific. Pericholecystic fluid is often seen but it is not nonspecific as well.
Layering of dense fluid within the gallbladder may be an indication of intraluminal hemorrhage (figure 33.), although milk of calcium or excretion of intravenous contrast media from prior CT studies are pitfalls that may cause similar findings. Bile duct injury can result in free fluid, or intrahepatic bile collections [50].
9. Bowel and mesenteric injuries
Are depicted in 3-5% of blunt abdominal trauma patients at laparotomy [51-53], and are the third most common type of injury from blunt trauma to abdominal organs.
Delayed diagnosis of bowel and mesenteric injuries results in increased morbidity and mortality, usually because of haemorrhage or peritonitis that leads to sepsis. Three basic mechanisms may cause bowel and mesenteric injuries of blunt trauma: Direct force may crush the gastrointestinal tract; rapid deceleration may produce shearing force between fixed and mobile portions of the tract; and a sudden increase in intraluminal pressure may result in bursting injuries.
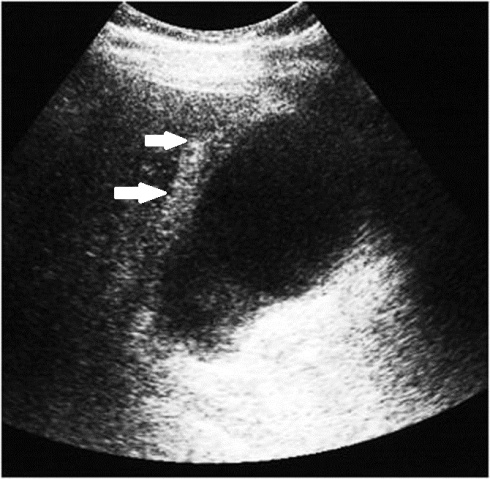
Multidetector CT is the most powerful tool in detecting abdominal traumatic injuries and is commonly used in evaluation of mesenteric and hollow organs lesions. It is more sensitive and specific than abdominal US.
Numerous CT signs have been described. The main goal in evaluating these signs is to distinguish significant bowel and mesenteric injuries that require surgical intervention from those that can be managed non surgically.
Helical CT scanning is very accurate in determining the need for surgical exploration in gastric injuries. However, it is less accurate in predicting the need for surgical exploration in mesenteric injuries alone
9.1. Bowel injury
Some findings are specific to bowel injury that are: bowel wall discontinuity, extraluminal contrast material and extraluminal air. Gas originating from a bowel rupture usually accumulates in locations deep to the anterior abdominal wall and may be seen also in the porta hepatis, mesentery or mesenteric veins, and portal vein.
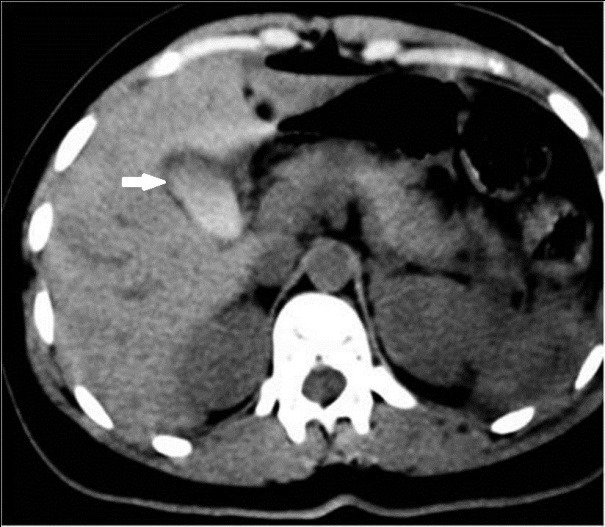
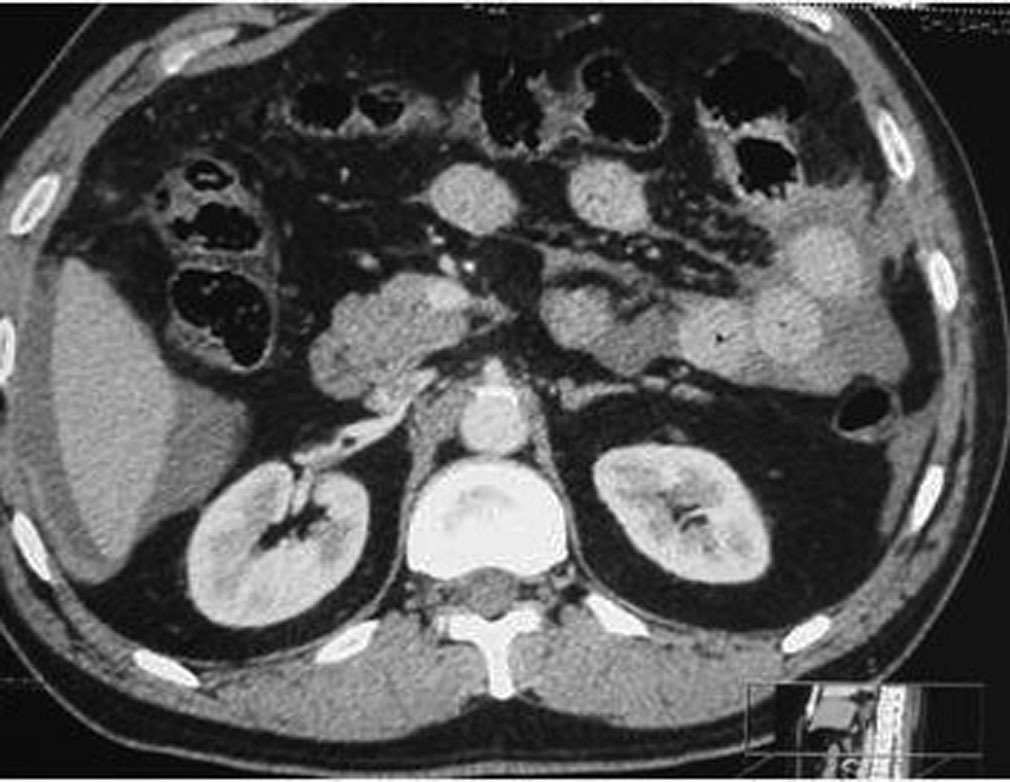
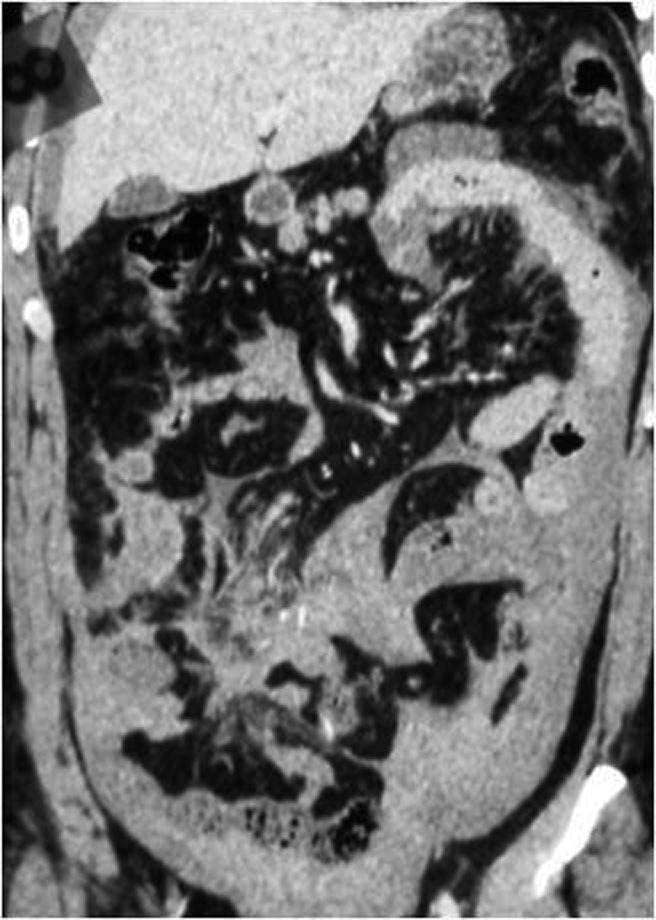
Some patterns are not specific: bowel wall thickening, abnormal bowel wall enhancement) and mesenteric foci of fluid or fat stranding may be secondary to bowel injury alone (Figure 34., 35.). Retroperitoneal air is seen with duodenal injury (Figures 36., 37., 38.) or the ascending or descending colon injury. Pancreatic transection should suggest duodenal injury.
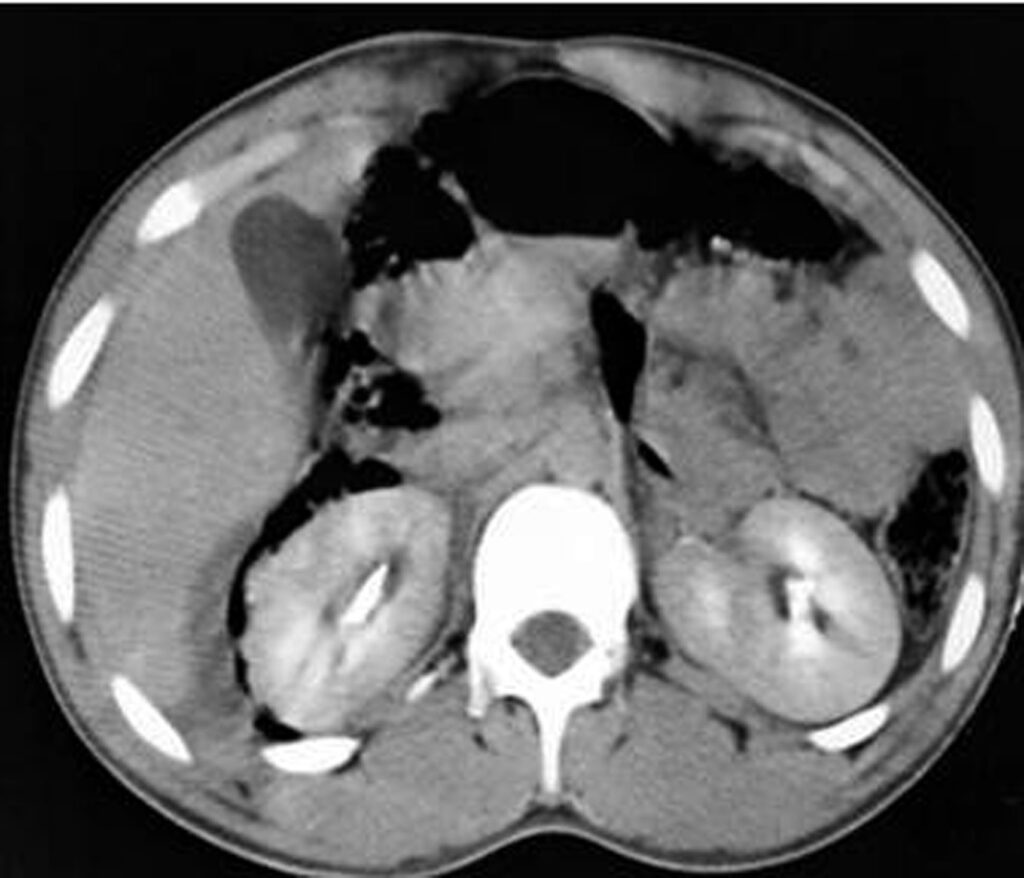
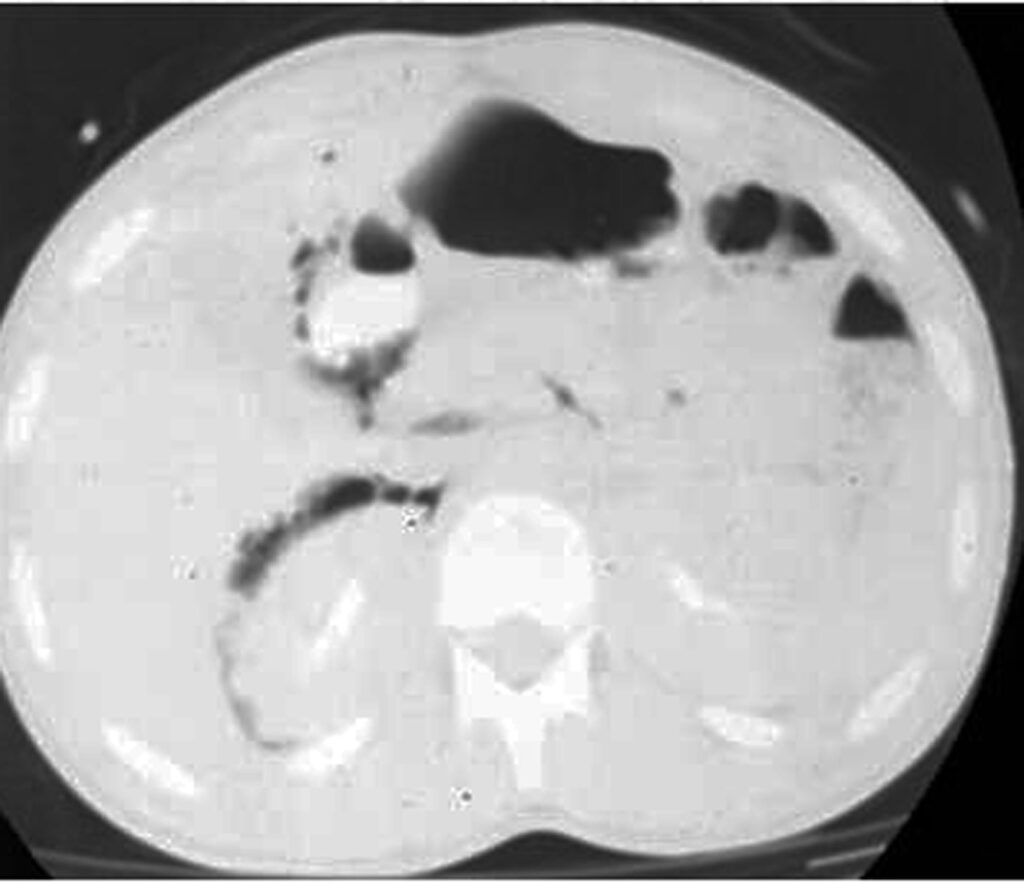
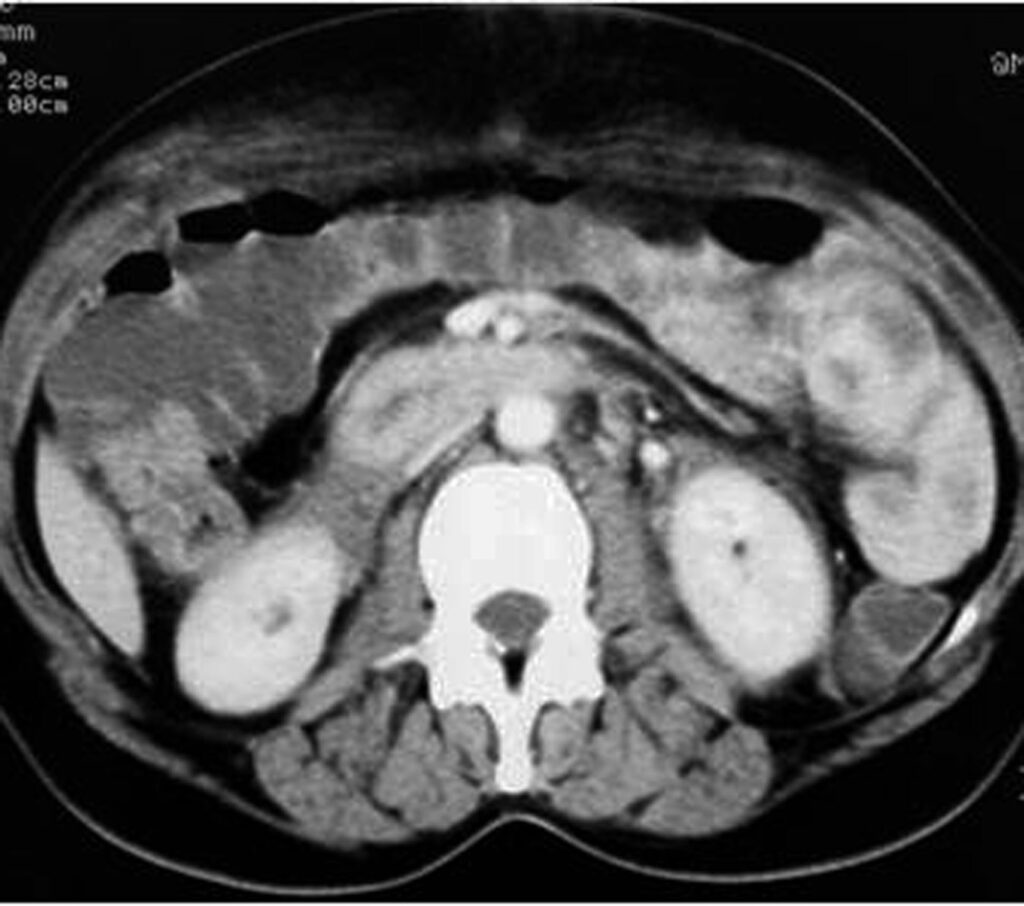
9.2. Isolated mesenteric injuries
Significant mesenteric injuries include active mesenteric bleeding, disruption of the mesentery, and mesenteric injury associated with bowel ischemia. An isolated mesenteric hematoma is considered non significant [51, 54].
Acute bleeding may occur from the small mesenteric vessels, and signs of intra-peritoneal blood loss make early laparotomy imperative. Injuries to the main mesenteric vessels resulting in acute haemorrhage are rare; Bowel infarction may result from either mesenteric tears with disruption of blood vessels or mesenteric vessel thrombosis, either venous or arterial: Mesenteric extravasation. This sign has a specificity of 100% for the diagnosis of significant mesenteric injury, but it was seen in only nine (17%) of 54 patients with bowel and mesenteric injuries inding of mesenteric extravasation is usually an indication for urgent lap (Figure 39., 40., 41.).
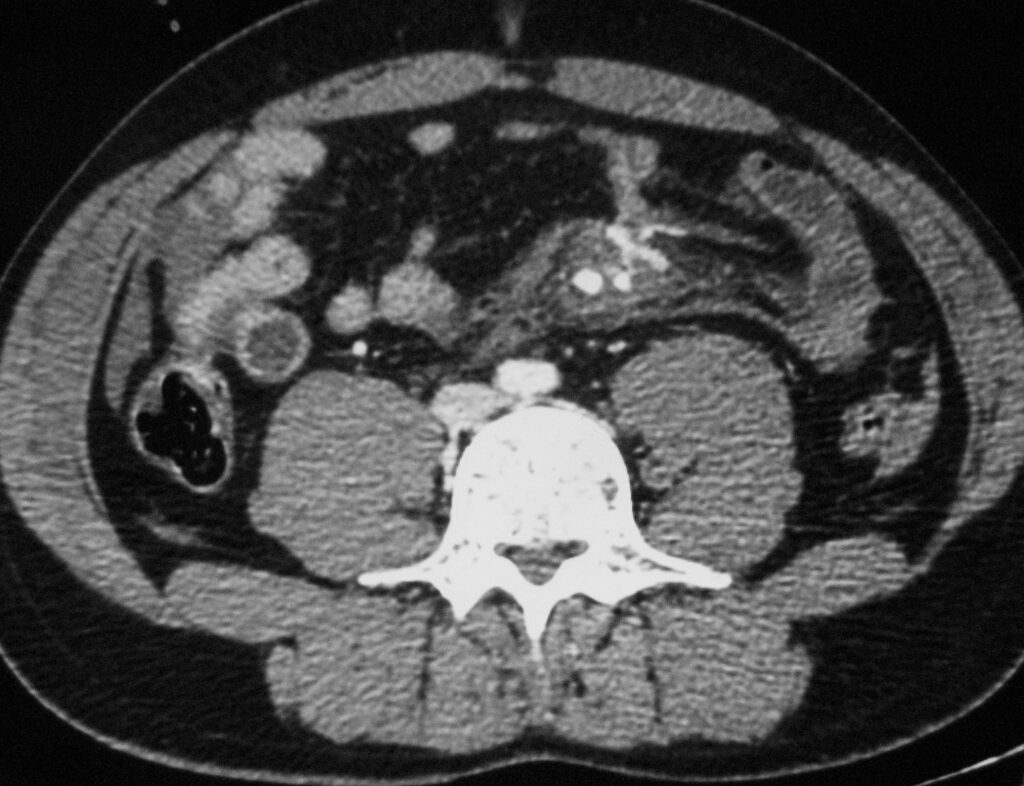
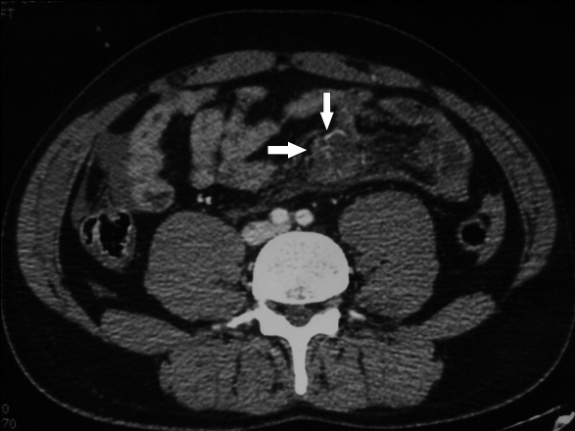

9.2.1. Mesenteric vascular beading
This feature appears on CT images as an irregularity in mesenteric vessels; like mesenteric extravasation of contrast material, it is indicative of vascular injury.
Termination of mesenteric vessels. Abrupt termination of a mesenteric artery or vein is also an indication of vascular injury.
Less specific findings as Mesenteric infiltration may indicate mesenteric injury with or without bowel wall injury. Mesenteric Hematoma seen as a well-defined mesenteric hematoma indicative of laceration of a mesenteric vessel [54].
10. Colonic injury
Compression of the upper abdomen caused by a steering wheel or lap-type seat belts appears to predispose patients to colonic injury.
The transverse colon, sigmoid and caecum portions are the most common sites of injury.
CT findings are intramural hematoma especially on the transverse colon, avulsion of the mesentery, full thicknes- laceration, transaction and devascularisation are seen in injuries of ascendin and descending colon [55].
11. Gastric traumatic injuries
Are rare, secondary to penetrating or blunt trauma. The incidence of blunt gastric trauma is estimated between 0.4% and 1.7% of all abdominal traumas [51, 52].
Blunt trauma lesions are more often the consequence of a high velocity impact involving the epigastric region in post-meal phase, since an important condition to occur is gastric fullness [53, 54]. Trauma may produce a gastric rupture, more frequently observed at the fundus (figures 42, 43, 44).



As in traumatic injuries of other abdominal organs, peritoneal fluid indicates a relevant lesion, mainly if it is bloody. The gas content of the stomach usually plays a protective role; sometimes it can dissect the mucosal layer and pass into the gastric veins. In these cases, portal pneumatosis may happen [56]. It is important to be aware of this condition because it is possible to misinterpret the pneumatosis as a consequence of an intestinal infarction due to the traumatic shock. It is also very important to play attention to gas considered that pneumoperitoneum is observed in almost all patients having a complete gastric rupture [50].
CT findings in intestinal perforation can be subtle and nonspecific. Wall thickening, wall discontinuity, extraluminal air, and mesenteric hematoma (figures 45, 46) are reasonably specific CT signs. The presence of a moderate to large volume of intraperitoneal fluid without visible solid organ injury is an important sign.


12. Diaphragmatic injury
The diaphragm may be injured by penetrating or blunt trauma. Diaphragmatic breach without visceral injury or herniation may be difficult to detect due to a paucity of clinical signs and herniation may be misdiagnosed following the wrong interpretation of chest radiology. If not recognized there is a considerable risk of late morbidity and mortality.
Plain chest radiography is the first technique to prefer. When performed immediately after the accident, it presents a suspicion about the diagnosis of a ruptured diaphragm in only.20–34% of the cases [57-59]. Diaphragmatic injury is shown as a soft-tissue opacity, containing visceral gas in the thorax, which is pathognomonic of diaphragmatic hernia). It also reveals associated rib fractures and haemopneumothorax. Although the strangulated bowel presents as intrathoracic air-fluid levels, the perforated bowel presents as pneumothorax. If there is a bowel obstruction, plain radiography of the abdomen shows air-fluid levels with abdominal distension [60]. The detection of a nasogastric tube above the left hemidiaphragm is a possible imaging feature.
CT examination of abdomen and thorax is a very useful and reliable tool in the evaluation of diaphragmatic injury. Multiplanar reformations are expected to improve sensitivity. CT can demonstrate findings consistent with diaphragmatic injury, such as diaphragmatic discontinuity, thickened diaphragm signs, intrathoracic herniation of abdominal contents, and waist-like constriction of abdominal viscera (the ‘collar sign’) [60-62], (Figures 47., 48.). An abnormal hepatic location depicted on axial CT can be considered as a potentially indirect sign of right diaphragm rupture with liver herniation. The so-called “dependent viscera” sign (when the upper one third of the liver abuts the posterior right ribs or whether the bowel or stomach lays in contact with the posterior left ribs) is nearly 100% specific [63]. The diaphragm itself may be obscured by hemothorax or hemoperitoneum.


13. Pelvic trauma
13.1. Arterial bleeding in pelvic trauma
Vascular injuries are a major source of morbidity and mortality in patients with blunt pelvic trauma. Bleeding is usually of venous origin. However, in 10%-20% of the patients, hemodynamic instability is associated with arterial hemorrhage. Mortality of up to 50% has been reported despite effective control of bleeding.
Pelvic CT angiography is useful in assessing vascular injuries and provides a complete study of bone, visceral and especially vascular lesions. Contrast-enhanced CT has been reported to be an accurate, noninvasive technique for identifying ongoing arterial hemorrhage in patients with pelvic fractures [64-68].
MDCT angiography study technique is able to identify the presence of active bleeding with the possibility in some cases of defining the source of the blood loss with a sensitivity of 66%– 90%, a specificity of 85%–98%, and an accuracy of 87%–98% being reported [66-67, 69-70]. High-quality multiplanar reconstructions provide a reliable vascular map of the anatomical structures (Figures 49., 50.). This technique is highly predictive of arterial injury that will require angiographic embolization [67], (Figures 51., 52.). The most important differential diagnosis is the clotted blood from which it is distinguished by measuring CT attenuation. Active bleeding shows higher attenuation.




In patients with pelvic trauma, the arterial vessels most frequently injured are the branches of the internal iliac artery, whereas the external iliac artery is less frequently involved.
The detection of contrast material extravasation on CT scans facilitates urgent angiography and subsequent transcatheter embolisation which are the most effective methods for controlling ongoing arterial bleeding and can be life-saving [71].
14. Place of interventional radiology in the management of trauma abdominals
Hemostasis and revascularization. Hemorrhagic lesions can be divided into visceral and vascular injuries pure. They may benefit from percutaneous embolization techniques, Dissections and traumatic thrombosis benefit from percutaneous revascularization due to recent technical advances [72].
14.1. Interventional radiology of hemostasis
14.1.1. Principles and technique
14.1.1.1. General principles
When the diagnosis of traumatic hemorrhagic parenchymal lesion and / or vascular was posed hemostasis is obtained by percutaneous embolization via a catheter angiography The technique is significantly different of embolization techniques for tumor or arteriovenous malformation by answering threecrucial points:
- The temporary embolization using absorbable material is usually sufficient to generate the local formation of thrombus. The recanalization of the occluded vessel secondary is not a problem.
- Vascular occlusion must always be performed at the site colitis.
- Embolization does not cause tissue damage, or at least minimally.
14.1.1.2. Vascular access
The percutaneous access is usually right or left femur. The use of bony landmarks under fluoroscopy or ultrasound guidance are quickly implemented in case of difficulty puncture order not to waste time on vascular access. If the patient already has an arterial access we can “take” it as an access. The use of a vascular introducer (désilet) 5-French is very useful to allow the rapid exchange catheter. In large pelvic trauma or major skin and muscle deformities, a brachial access is necessary [72-73].
14.1.1.3. Catheterization
If the patient has already received a CT scan with injection of iodinated contrastThe catheterization will be immediately on known or suspected bleeding sites. Without scanner, the aortography face must be systematic to guide the selective catheterization. negativity of aortography does not rule out the presence of hemorrhagic lesions that onlyselective series can deny. The most commonly used catheters are pre-formed type Cobra and Simmons. Caliber 4 or 5-French, they can usually make the diagnosis and treatment. In case of difficult catheterization or to embolize very selectively, Recent advances in materials make available micro-catheter 2 or 3-French highly efficient and capable of delivering micro-emboli [72-73].
14.1.1.4. Emboli and embolization
The Curaspon®, temporary embolus type animal gelatin which disappears within three weeks the best embolus Fragments of variable size and shape are used according to the habits of each; Only the use of this product powder is clearly inadvisable. Indeed, as the particles PVA (polyvinyl alcohol), who also have the drawback of being definitive emboli, powder provides a very distal embolization, which has no interest in this context and can generate extensive visceral infarcts and abscesses. The coils, final emboli formed by coiling optionally covered with thrombogenic fibers, are widely used in certain territories or failure of Curaspon® may occur in case of major disruption of hemostasis. The use of biological glue Histo-Acryl ®-type (n-butylcyanoacrylate) which polymerizes in contact with basic environments such as water or blood is possible in case of difficulties in obtaining satisfactory embolization with other emboli. It should not be used in first intention. Final bolus, its handling requires great skill and its use in the context of trauma that is the subject of rare publications.
14.1.2. Embolization of visceral injuries
Most teams currently recognize conservative nonsurgical treatment as the standard treatment of hemodynamically stable patients the exploration and surgical treatment remain the reference of unstable patients. It is within this context of conservative treatment that is positioned interventional radiology, the indications are in many assessment teams without well-defined consensual attitude to this day.
14.1.2.1. Splenic injury
Conservative treatment, strict bed rest and Surveillance, is supposed to be the reference, we are surprised by a great heterogeneity of findings with failure rates between 2 and 52%. Moreover, some studies have shown that a hemoperitoneum greater than 300 ml, a high grade lesion scannographic and / or the presence of a leak active contrast or a« blush » scanner to be risk factors for failure. These patients would thus theoretically good candidates for further treatment by embolization. Once the indication for embolization posed, remains the choice of technique, currently being discussed in the literature: selective embolization of vascular lesions viewed or proximal embolization of the splenic artery trunk by coils. The first one, theoretically longer would be associated with more frequent and extensive splenic infarction. The second concept is to reduce the intra-splenic pression to allow hemostasis while leaving the possibility of a resumption of the splenic blood supply by the short gastric vessels. The addition of embolization achieves failure rates below 10% among patients with high-grade lesions. Remains the poorly known problem of residual splenic function after embolization [74-76].
14. 1.2.2. Liver injury
As for the spleen, indications of hepatic arterial chemoembolization remain to be defined. Both indications are currently the most common: the persistent déglobulisation in a traumatized liver and the detection of contrast leakage or intraparenchymal blush on CT. Embolization should be here as selective as possible. The series of the literature report success rates of 90-100% and low morbidity [74, 77, 78].
14.1.2.3. Kidney injury
The arteriovenous embolization was reported in cases of extravasation of contrast, of arteriovenous fistula or pseudoaneurysm with a very high efficiency. The embolization should be as selective as possible to preserve as much renal parenchyma as possible, most often using microcatheters and microcoils. In the extended or proximal forms, the embolization of renal artery trunk is possible,, To control bleeding and avoid nephrectomy of hemostasis, act often dreaded by surgeons [79-80].
14.1.3. Vascular injuries “pure”
14.1.3.1. Pelvic injuries
Conducted immediately in a patient carrying an unstable pelvic fracture or secondarily after visualization of a pelvic active leak, the embolization of internal iliac territories will be selective if possible (Figure 51., 52.)
The involved branches are in decreasing order of frequency: superior gluteal, lateral sacral, iliolumbar, obturator, inferior gluteal.The selective catheterization, however, can be long. And the proximal internal iliac embolization unilateral or bilateral must be the first option in the very unstable patients. Conventionally carried out using Curaspon ®, the embolization may be supplemented with coils in case of bleeding disorders after massive transfusion. The internal iliac embolization is now a mature technology, efficient (90-100% control of bleeding) and safe [72, 81].
14.1.3.2. Retroperitoneal injuries
The lumbar and iliolumbar arteries are most often involved. The Bleeding may also come from other arteries: intercostal, inferior phrenic, adrenal, pancreaticoduodenal. Two elements are crucial when we are led to embolize thoracolumbar territories:
- The origin of the anterior spinal artery must be tracked and the embolization carried out downstream thereof where appropriate.
- The levels metameric arteries above and underlies a lumbar artery should be embolized to prevent further bleeding by the physiological interlombaires anastomoses [72, 81].
14.2. Interventional radiology of revascularization
The introduction of stents has been described as in traumatic dissections of the renal artery in clinical cases or small series. This is made possible by the knowledge and technical developments derived from acquired coronary stenting. The period of treatment remains unclear: the theoretical threshold of 6 hours of ischemia could be the rule but recent work has shown the absence of renal functional benefit for revascularization (endovascular or surgical) beyond 4 hours of warm ischemia.
15. Conclusion
Beyond the diagnosis, the radiologist offers, through technological advances, of minimally invasive treatment options, fast, available, efficient and more mature. Thus, progress of interventional radiology in trauma residing longer in the indications progress that in the techniques progress between the “all surgical” and “all conservative”, the various interventional techniques have certainly an important place to take.
16. Gunshot wounds
Surgical exploration of abdominal gunshot wound victims has been the standard of care for the greater part of the last century, The accumulating evidence demonstrate, however, that taking all abdominal gunshot wound victims to laparotomy leads to a negative or non-therapeutic procedure in 15% to 25% of cases [82-85].
The management of hemodynamically stable abdominal gunshot wound victims has been changing in the last few years and has been gradually replaced by a conservative strategy. Diagnostic imaging methods are providing information which could help with a more appropriate treatment decision. Abdominal plain radiographies are used to search for pneumoperitoneum and to identify the location and number of retained projectiles. Ultrasonography is less used in penetrating trauma. The role of CT in evaluating hemodynamically stable blunt abdominal trauma patients is well established, and CT became the imaging modality of choice in this situation [36]. Lesions may involve solid and/or hollow organs, the urinary bladder, vessels, diaphragm and bones. MDCT is safely used to determine projectile trajectory and likely injuries (figure 53., 54., 55).
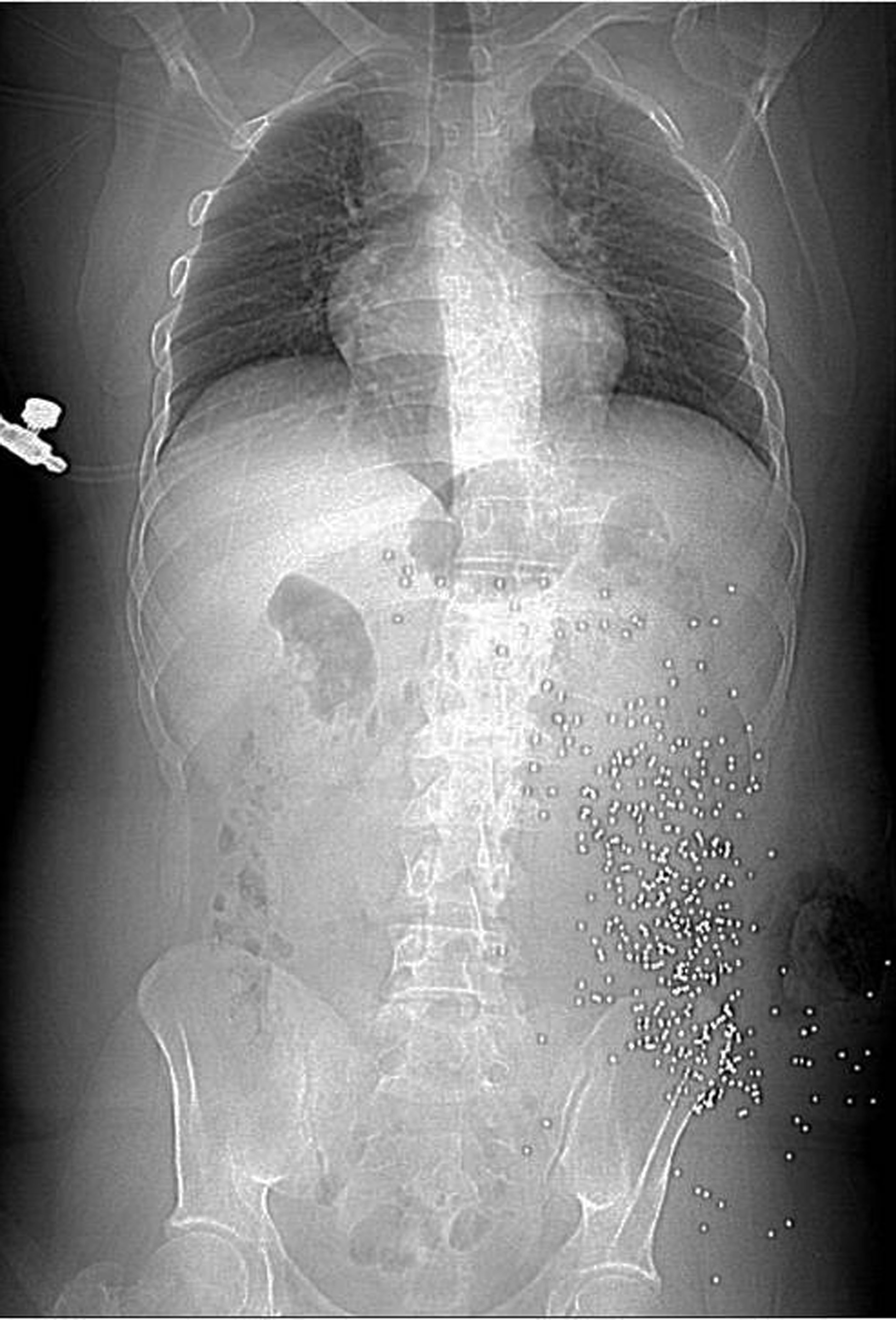


17. Conclusion
Abdominal trauma represents an important part of daycare activity in radiology. Nonsurgical treatment has become the standard of care in hemodynamically stable patients with abdominal trauma as a result of exhaustive and rigorous assessment of injury lesions by imaging. US is a non invasive readily available method of detection of free fluid in trauma patient; the fast exam looks for intraperitoneal and intrapericardial anechoic fluid and allows selection of patients for CT. CT is the technique of choice for initial examination of hemodynamically stable patients after abdominal trauma because it is highly sensitive, specific and accurate in detecting the presence or absence of injury and defining its extend.
References
- 1.Ultrasonography for the diagnosis of intraperitoneal free air in chest-abdominal-pelvic blunt trauma and critical acute abdominal pain.Arch Surg. 2009144213741
- 2.CokkinosD.AntypaE.StefanidisK.TserotasP.KostarasV.ParlamentiA.TavernarakiK.PiperopoulosP. N.Contrast-enhanced ultrasound for imaging blunt abdominal trauma- indications, description of the technique and imaging review. Ultraschall Med. 2012331607
- 3.Branney SW, Wolfe RE, Moore EE. et al.Quantitative sensitivity of ultrasound in detecting free intraperitoneal fluid. J Trauma. 199539375380
- 4.Goodman CS, Hur JY, Adajar MA et al.How well does CT predict the need for laparotomy in hemodynamically stable patients with penetrating abdominal injury? A review and meta-analysis. AJR Am J Roentgenol. 2009193432437
- 5.ShanmuganathanK.MirvisS. E.Boyd-KranisR.et al.Nonsurgical management of blunt splenic injury: use of CT criteria to select patients for splenic arteriography and potential endovascular therapy. Radiology. 20002177582
- 6.ShanmuganathanK.MirvisS. E.SherbournC. D.et al.Hemoperitoneum as the sole indicator of abdominal visceral injuries: a potential limitation of screening abdominal US for trauma. Radiology. 19992122423430
- 7.Wing VW, Federle MP, Morris JA Jr, et al.The clinical impact of CT for blunt trauma. AJR Am J Roentgenol. 198514511911194
- 8.ClarkT. J.CardozaS.KanthN.Splenictrauma.pictorialreview.ofcontrast-enhanced. C. T.findingsEmerg Radiol. 201118322734
- 9.EEMooreShackford. S. R.PachterH. L.Mc AninchJ. W.BDBrownerChampion. H. R.FlintL. M.GennarelliT. A.MAMalangoniRamenofsky. M. L.et al.Organinjury.scalingspleen.liverkidneyJ.Trauma1989291216646
- 10.TinkoffG.EspositoT. J.ReedJ.KilgoP.FildesJ.PasqualeM.MeredithJ. W.American Association for the Surgery of Trauma Organ Injury Scale I: spleen, liver, and kidney, validation based on the National Trauma Data Bank. J Am Coll Surg. 2008207564655
- 11.MarmeryH.ShanmuganathanK.AlexanderM.et al.Optimization of selection for nonoperative management of blunt splenic injury: comparison of MDCT grading systems. AJR. 200718914211427
- 12.MatthesG.StengelD.SeifertJ.et al.Blunt liver injuries in polytrauma: results from a cohort study with the regular use of whole-body helical computed tomography. World J Surg. 20032711241130
- 13.RomanoL.GiovineS.GuidiG.TortoraG.CinqueT.RomanoS.Hepatictrauma. C. T.findingsconsiderationsbased.onour.experiencein.emergencydiagnostic.imagingEur J Radiol. 20045015966
- 14.Croce MA, Fabian TC, Menke PG, et al.Nonoperative management of blunt hepatic trauma is the treatment of choice for hemodynamically stable patients: results of a prospective trial. Ann Surg. 1995221744755
- 15.Pachter HL, Spencer FC, Hofstetter SR, Liang HG, Coppa GF.Significant trends in the treatment of hepatic trauma: experience with 411 injuries. Ann Surg. 1992215492500
- 16.ShanmuganaK.MirvisS. E. C. T.evaluationof.theliver.withacute.blunttrauma.Crit Rev Diagn Imaging. 19953673113
- 17.Mirvis SE, Whitthey NO, Vainwright JR, Gens DR.Blunt hepatic trauma in adults: CT base classification and correlation with prognosis and treatment. Radiology 19891712732
- 18.Croce MA, Fabian TC, Kudsk KA, Baum SL, Payne LW, Mangiante LC, et al. AAST organ injury scale: correlation of CT graded liver injuries and operative findings.J Trauma. 199131680612
- 19.YoonW.JeongY. Y.KimJ. K.SeoJ. J.LimH. S.ShinS. S.KimJ. C.JeongS. W.ParkJ. G.KangH. K. C. T.inblunt.livertrauma.Radiographics. 200525187104
- 20.MieleV.AndreoliC.de CiccoM. L.AdamiL.DavidV.Hemoperitoneum associated with liver bare area injuries: CT evaluation. Eur Radiol 200212765769
- 21.PolettiP. A.MirvisS. E.ShanmuganathanK.KilleenK. L.ColdwellD. C. T.criteriafor.managementof.bluntliver.traumacorrelation.withangiographic.surgicalfindings.Radiology. 2000216418427
- 22.HagiwaraA.MurataA.MatsudaT.MatsudaH.ShimazakiS.The efficacy and limitations of transcatheter embolization for severe hepatic injury. J Trauma. 20025210911096
- 23.Fang JF, Chen RJ, Wong YC, et al.Pooling of contrast material on computed tomography mandates aggressive management of blunt hepatic injury. Am J Surg. 1998176315319
- 24.Fang JF, Chen RJ, Wong YC, et al.Classification and treatment of pooling of contrast material on computed tomographic scan of blunt hepatic trauma. J Trauma. 20004910831088
- 25.Wong YC, Wang LJ, See LC, et al.Contrast material extravasation on contrast-enhanced helical computed tomographic scan of blunt abdominal trauma: its significance on the choice, time, and outcome of treatment. J Trauma. 200354164170
- 26.ShanmuganathanK.MirvisS. E. C. T.scanevaluation.ofblunt.hepatictrauma.Radiol Clin North Am. 19983639941
- 27.SicaG.BocchiniG.GuidaF.TangaM.GuaglioneM.ScaglioneM.Multidetector computed tomography in the diagnosis and management of renal trauma. Radiol med. 2010115936949
- 28.RazaliM. R.AAAzianAmran. A. R.AzlinS.Computed tomography of blunt renal trauma. Singapore Med J. 2010
- 29.Lee YJ, oh SN, Rha SE, Byun JY. Renal trauma. Radiol clin Nam.2007
- 30.AlonsoR. C.BorruelNacenta. S.DiezMartinez. P.ASGuerreroFuentes. C. G.Kidneyin.DangerC. T.Findingsof.BluntPenetratingRenal.TraumaRadio.RadioGraphics. 20092920332053
- 31.GuptaA.StuhlfautJ. W.FlemingK. W.LuceyB. C.SotoJ. A.Blunt trauma of the pancreas and biliary tract: a multimodality imaging approach to diagnosis. Radiographics 2004245138195
- 32.LinsenmaierU.WirthS.ReiserM.KörnerM.Diagnosis and classification of pancreatic and duodenal injuries in emergency radiology. Radiographics 20082861591602
- 33.Cirillo RL Jr, Koniaris LG.Detecting blunt pancreatic injuries. J Gastrointest Surg. 2002645879
- 34.PatelS. V.SpencerJ. A.El -HasaniS.SheridanM. B.Imaging of pancreatic trauma. Br J Radiol. 199871985990
- 35.Venkatesh SK, Chin Wan JM. CT of blunt pancreatic trauma-a pictorial essay.Eur J Radiol. 200867311320
- 36.ShanmuganathanK.Multi-detectorrow. C. T.imagingof.bluntabdominal.traumaSemin Ultrasound CT MR. 200425180204
- 37.RekhiS.AndersonS. W.RheaJ. T.SotoJ. A.Imaging of blunt pancreatic trauma. Emerg Radiol. 2010171139Epub 2009 Apr 25.
- 38.Wong YC, Wang LJ, Lin BC et al. CT grading of blunt pancreatic injuries: prediction of ductal disruption and surgical correlation. J Comput Assist Tomogr. 199721246250
- 39.Phelan HA, Velmahos GC et al.An evaluation of multidetector computed tomography in detecting pancreatic injury: results of a multicenter AAST study. J Trauma. 20096636416discussion 646-7.
- 40.Lane MJ, Mindelzun RE, Sandhu JS et al. CT diagnosis of blunt pancreatic trauma: importance of detecting fluid between the pancreas and the splenic vein.AJR Am J Roentgenol. 1994163833835
- 41.Bradley EL, Young PR, Chang MC et al.Diagnosis and initial management of blunt pancreatic trauma: guidelines from a multiinstitutional review. Ann Surg. 1998227861869
- 42.DuchesneJ. C.SchmeigR.IslamS.et al.Selective nonoperative management of low grade blunt pancreatic injury: are we there yet? J Trauma. 2008654953
- 43.BrestasP. S.KarakyklasD.GardelisJ.et al.SequentialC. T.evaluationof.isolatednon-penetrating.pancreatictrauma. J. O.JOP. 200675155
- 44.Anderson SW, Soto JA. Pancreatic duct evaluation: accuracy of portal venous phase 64 MDCT Abdom Imaging.2009345563
- 45.Wong YC, Wang LJ, Fang JF et al.Multidetector-row computed tomography (CT) of blunt pancreatic injuries: can contrast-enhanced multiphasic CT detect pancreatic duct injuries? J Trauma. 200864666672
- 46.Kim HS, Lee DK, Kim IW, et al.The role of endoscopic retrograde pancreatography in the treatment of traumatic pancreatic duct injury. Gastrointest Endosc. 2001544955
- 47.RagozzinoA.ManfrediR.ScaglioneM.et al.The use of MRCP in detection of pancreatic injuries after blunt trauma. Emerg Radiol. 2003101418
- 48.Matos C, Winant C, Deviere J. Magnetic resonance pancreatography. Abdom Imaging. 2001; 26:243-253.
- 49.GillamsA. R.KurzawinskiT.LeesW. R.Diagnosis of duct disruption and assessment of pancreatic leak with dynamic secretin-stimulated MR cholangiopancreatography. AJR Am J Roentgenol. 2006186499506
- 50.BroderJ.Diagnostic Imaging for the Emergency Physician, 2011578611
- 51.McCullough CJ.Isolated mesenteric injury due to blunt abdominal trauma. Injury. 7295298
- 52.Lassandro F, Romano S, Rossi G, Muto R, Cappabianca S, Grassi R. Gastric traumatic injuries: CT findings. Eur J Radiol. 2006; 59:349-54.
- 53.FuruyaY.YasuharaH.ArikiK.YanagieH.NakaS.NojiriT.et al.Hepatic portal venous gas caused by blunt abdominal trauma: is it a true ominous sign of bowel necrosis? Report of a case. Surg Today. 2002326558
- 54.BrodyJ. M.LeightonD. M.MurphyB. L.AbbottG. F.VaccaroJ. P.JagminasL.CioffiW. G. C. T.ofblunt.traumabowel.mesentericinjury.typicalfindings.pitfallsin.diagnosisRadiographics. 200020152536
- 55.VuNghiem. H.BrookeJeffrey. R.Jr MindelzunR. E. C. T.ofblunt.traumato.thebowel.mesenteryA. J.AJR 19931605358
- 56.BrofmanN.AtriM.EpidD.HansonJ. M.GrinblatL.ChughtaiT.RennemanF.Evaluation of Bowel and Mesenteric Blunt Trauma with Multidetector CT. RadioGraphics 20062611191131
- 57.NursalT. Z.UgurluM.KologluM.HamalogluE.Traumaticdiaphragmatic.herniasa.reportof. .casesHernia 200152591
- 58.Ramos CT, Koplewitz BZ, Babyn PS, Manson PS, Ein SH.What have we learned about traumatic diaphragmatic hernias in children? J Pediatr Surg 2000356014
- 59.SadeghiN.NicaiseN.De BackerD.StruyvenJ.Van GansbekeD.Right diaphragmatic rupture and hepatic hernia: an indirect sign on computed tomography. Eur Radiol 199999724
- 60.ErenS.KantarciM.OkurA.Imaging of diaphragmatic rupture after trauma. Clin Radiol. 200661646777
- 61.NchimiA.SzapiroD.GhayeB.WillemsV.KhamisJ.HaquetL.NoukouaC.DondelingerR. F.HelicalC. T.ofblunt.diaphragmaticrupture. A. J.AJR Am J Roentgenol. 200518412430
- 62.Chen HW, Wong YC, Wang LJ, Fu CJ, Fang JF, Lin BC.Computed tomography in left-sided and right-sided blunt diaphragmatic rupture: experience with 43 patients. Clin Radiol. 201065320612Epub 2010 Jan 4.
- 63.BerginD.EnnisR.KeoghC.FenlonH. M.MurrayJ. G.The”dependent.viscerasign.inC. T.diagnosisof.blunttraumatic.diaphragmaticA. J.AJR Am J Roentgenol. 20011775113740
- 64.YoonW.KimJ. K.JeongY. Y.SeoJ. J.ParkJ. G.KangH. K.Pelvic arterial hemorrhage in patients with pelvic fractures: detection with contrast-enhanced CT. Radiographics. 20042461591605
- 65.ShanmuganathanK.MirvisS. E.SoverE. R.Value of contrast-enhanced CT in detecting active hemorrhage in patients with blunt abdominal or pelvic trauma. AJR Am J Roentgenol. 19931616569
- 66.DSCervaMirvis. S. E.ShanmuganathanK.KellyI. M.PaisS. O.Detection of bleeding in patients with major pelvic fracture: value of contrast-enhanced CT. AJR Am J Roentgenol. 1996166131135
- 67.Stephen DJ, Kreder HJ, Day AC, et al.Early detection of arterial bleeding in acute pelvic trauma. J Trauma. 199947638642
- 68.Pereira SJ, O’Brien DP, Luchette FA, et al.Dynamic helical computed tomography scan accurately detects hemorrhage in patients with pelvic fracture. Surgery. 2000128678685
- 69.HagiwaraA.MinakawaK.FukushimaH.MurataA.MasudoH.ShimazakiS.Predictors of death in patients with life-threatening pelvic hemorrhage after successful transcatheter arterial embolization. J Trauma. 200355696703
- 70.Pereira SJ, O’Brien DP, Luchette FA, et al.Dynamic helical computed tomography scan accurately detects hemorrhage in patients with pelvic fracture. Surgery. 2000128678685
- 71.PintoA.NiolaR.TortoraG.PonticielloG.RussoG.Di NuzzoL.GagliardiN.ScaglioneM.MerolaS.StavoloC.MaglioneF.RomanoL.Role of multidetector-row CT in assessing the source of arterial haemorrhage in patients with pelvic vascular trauma. Comparison with angiography. Radiol Med. 2010115464867
- 72.VelmahosG.ToutouzasK.VassiliuP.etal. A.prospectivestudy.onthe.safetyefficacyof.angiographicembolization.forpelvic.visceralinjuries.J Trauma 2002533038
- 73.KishJ.KatzM.MarxM.HarrellD.HanksS.N-butyl cyanoacrylate embolization for control of acute arterial hemorrhage. J Vasc Interv Radiol. 20041568995
- 74.AlonsoM.BrathwaiteC.GarciaV.et al.Practice management guidelines for the nonoperative management of blunt injury to the liver and spleen. In: www.east.org2003
- 75.HaanJ.ScottJ.Boyd-KranisR.HoS.KramerM.ScaleaT.Admission angiography for blunt splenic injury: advantages and pitfalls. J Trauma. 20015111615
- 76.BessoudB.DenysA.Main splenic artery embolization using coils in blunt splenic injuries: Effects on the intrasplenic blood pressure. Eur Radiol. 20041417189
- 77.VelmahosG.ToutouzasK.RadinR.CjanL.DemetriadesD.Nonoperative treatment of blunt injury to solid abdominal organs. J Trauma 200313884451
- 78.HagiwaraA.YukiokaT.OhtaS.et al.Nonsurgical management of patients with blunt hepatic injury: efficacy of transcatheter arterial embolization. AJR 199716911516
- 79.VelmahosG.ToutouzasK.RadinR.CjanL.DemetriadesD.Nonoperative treatment of blunt injury to solid abdominal organs. J Trauma. 20031388445
- 80.DinkelH.DanuseR. H.TrillerJ.Bluntrenal.traumaminimally.invasivemanagement.withmicrocatheter.embolizationexperience.innine.patientsRadiology 200222372330
- 81.CookR.KeatingJ.GillespieI.The role of angiography in the management of haemorrhage from major fractures of the pelvis. J Bone Joint Surg Br. 20028417882
- 82.NanceF. C.WennarM. H.JohnsonL. W.IngramJ. C.Jr CohnI.Jr Surgical judgment in the management of penetrating wounds of the abdomen: experience with 2212 patients. Ann Surg. 19741795639646
- 83.LoweR. J.JDSalettaRead. D. R.RadhakrishnanJ.MossG. S.Should laparotomy be mandatory or selective in gunshot wounds of the abdomen? J Trauma. 19771712903907
- 84.Renz BM, Feliciano DV.Unnecessary laparotomies for trauma: a prospective study of morbidity. J Trauma. 1995383350356
- 85.Henderson VJ, Organ CH Jr, Smith RS. Negative trauma celiotomy. Am Surg.1993596365370
